Molecular Toxicology Group

The Mechanistic Toxicology Group (mTOX) is dedicated to understanding how chemical exposure disrupts biology. Our main areas of research include the development of New Approach Methods (NAMs) for Developmental and Adult Neurotoxicity Testing (DNT and ANT), integration of multiple, zebrafish-based endpoints to predict toxicity outcomes, the use of molecular approaches to reveal causal mechanisms by which chemical exposure affects neurodevelopment, the investigation of chemical-microbiome interactions that influence host health, and NAM development to assess the effect of chemical mixtures on behavior and enteric nervous system development.
Build Developmental Neurotoxicity (DNT) and Adult Neurotoxicity (ANT) NAMs
The developing nervous system is particularly sensitive to chemical exposure and there is heightened public concern linking the rise in children’s neurodevelopmental impairments, including learning disabilities, to environmental chemicals. From an ecological perspective, ~16% of chemicals detected in multiple EU water sampling studies have neurotoxic modes of action. Despite the link between chemical exposure and adverse neurodevelopmental outcomes, from among an estimated 350.000 chemicals in commerce, data from less than 200 OECD rodent neurotoxicity tests are available. To solve the gap in testing, we need to radically change the way we assess the potential for chemicals to harm the developing nervous system. Cellular assays fail to recapitulate complex neurodevelopmental endpoints captured in OECD rodent tests including behavior, learning, and memory. mTox builds, evaluates, and validates zebrafish embryo-based NAMs for complex behavioral endpoints including non-associative habituation learning and memory retention, and build fingerprinting systems to reveal putative chemical mode of action information. Funded by the Partnership for the Assessment of Risk from Chemicals (PARC) and UFZ CITE. In the PANDORA project, funded by the European Food Safety Authority, we are modifying our behavior battery, coupled with high-content imaging of transgenic zebrafish labeling dopaminergic cells, to understand how dopaminergic cell loss is linked to behavioral manifestations observed upon exposure to reference chemicals and pesticides used in Europe.
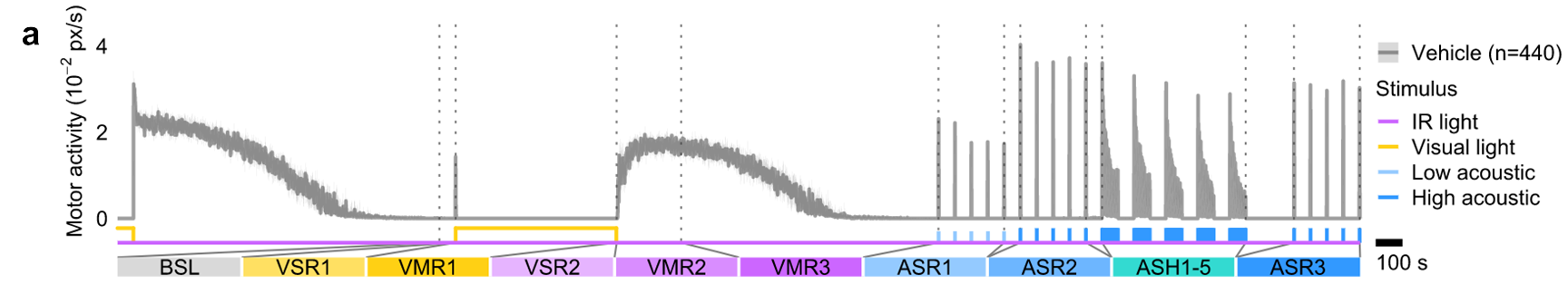 Figure: We developed a novel zebrafish embryo behavior assay battery for the identification of neurotoxic chemicals. The battery is comprised of sequential behavior assays that quantify visual and acoustic startle responses (VSRs, ASRs), visual motor responses (VMRs), acoustic startle habituation (ASH), potentiation of habituation, and memory retention of acoustic stimuli. We use the assay to identify chemicals that disrupt complex behaviors in early life stage zebrafish.
Figure: We developed a novel zebrafish embryo behavior assay battery for the identification of neurotoxic chemicals. The battery is comprised of sequential behavior assays that quantify visual and acoustic startle responses (VSRs, ASRs), visual motor responses (VMRs), acoustic startle habituation (ASH), potentiation of habituation, and memory retention of acoustic stimuli. We use the assay to identify chemicals that disrupt complex behaviors in early life stage zebrafish.
mTox DNT and ANT NAM Team: David Leuthold, Nadia Herold, Renee Owen, Jana Raab, Julia Spath, Stefan Scholz, Tamara Tal
Discover chemical mode of action
Zebrafish embryo behavior data has yet to be used for human and ecological risk assessment. One reason is a lack of confidence that chemical-dependent changes in zebrafish behavior represent developmentally or acutely neurotoxic endpoints that are translationally relevant. One strategy that can build confidence in the use of these data is a better understanding of underlying mechanisms that drive behavior effects. mTox seeks to causally describe chemical mode of action to generate regulatory confidence that a chemical pollutant is likely to disrupt neurodevelopment across taxa, including humans. Funded by the Partnership for the Assessment of Risk from Chemicals (PARC) and UFZ CITE.
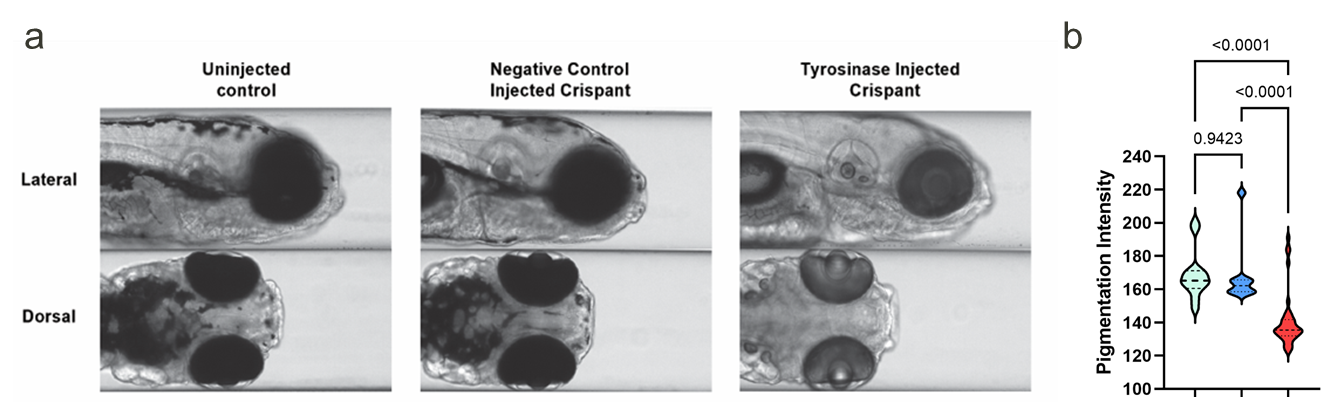 Figure: CRISPR/Cas9-based gene editing of the tyrosinase gene caused significant reductions in pigmentation (light blue=uninjected control; blue=negative control crispant; red=tyrosinase crispant). This strategy is applied to test whether genes and pathways are required for chemical-dependent effects on behavior.
Figure: CRISPR/Cas9-based gene editing of the tyrosinase gene caused significant reductions in pigmentation (light blue=uninjected control; blue=negative control crispant; red=tyrosinase crispant). This strategy is applied to test whether genes and pathways are required for chemical-dependent effects on behavior.
mTox Mechanism Team: Sebastian Gutsfeld, Nicole Schweiger, David Leuthold, Renee Owen, Stefan Scholz, Tamara Tal
Integration of high-content imaging and automated behavior analysis in zebrafish embryo assays to understand and predict the effects of chemicals
Changes in embryonic development and behavior endpoints represent common endpoints in zebrafish embryo assays. However, their analysis is not fully explored due to the lack of procedures for automated and unbiased assessment of changes provoked by chemicals. Therefore, we have developed routines based on model training, imaging and video frame subtractions to establish a detailed and reproducible assessment of morphological, functional and behavioural phenotypes. Supported by own open-source software developments such as FishInspector or EmbryoMotion we apply the high-content effect assessment for the prediction of human (developmental) or environmental toxicity and to diagnose mode of actions based on effect patterns. A central aspect of our analysis is a critical analysis of specificity of the effects, by comparison to predicted, hydrophobicity-driven baseline toxicity. Furthermore, in order to understand the role of toxicokinetics for the manifestation of effects we collaborate with the department of Environmental Analytical Chemistry and Cell Toxicology for internal exposure assessment.
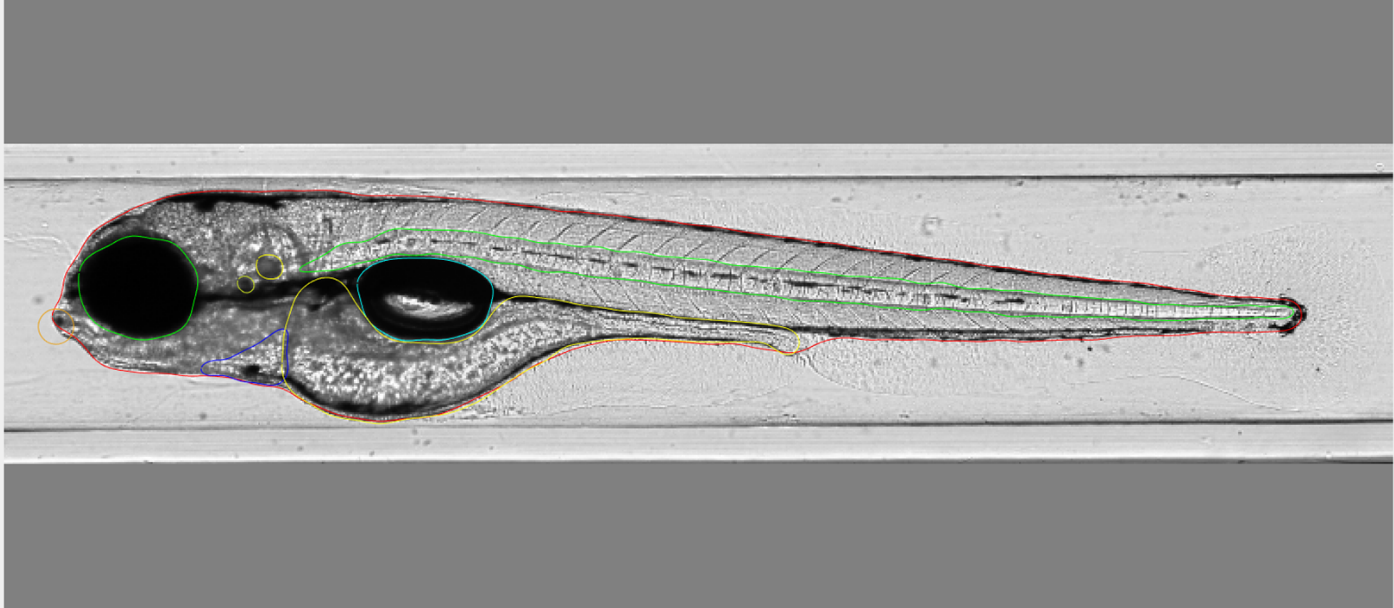 Figure: Automated imaging in combination with image annotation is used for a quantitative assessment of phenotypes in in zebrafish embryos exposed to chemicals.
Figure: Automated imaging in combination with image annotation is used for a quantitative assessment of phenotypes in in zebrafish embryos exposed to chemicals.
mTox zebrafish high content team/people: Muhammad Arslan Aslam, Ann-Kathrin Kolbitz (Alumni from Jan 2025), Vanessa Saalmann, Bingxu Chen (from Jan 2025), Gloria Chidibiere Ajugwo, Emmanuel Chukwu
Understand chemical-microbiome interactions that affect host health
The intestinal microbiome harbors the capacity to influence the toxicokinetics and toxicodynamics of xenobiotic exposures. It is widely understood that exposure to exogenous chemicals disrupts the community structure of host-associated microbes and several reports show that chemical-selected microbiomes biotransform environmental chemicals. A major knowledge gap relates to whether xenobiotic-induced effects on microbial ecology and biotransformation causally alter the toxicity of environmental chemicals to the host organism. Until this question is answered, the microbiome will not be considered in risk assessment strategies. mTox moves the science forward to illuminate the mode of action in microbiome-mediated toxicity using the three-colonization cohort system comprised of axenic (i.e., microbe-free), conventionalized, and conventionally colonized zebrafish. Funded by Neuro-Xeno-Microbiome and UFZ CITE MibiTox Consortium.
 Figure: This image depicts an axenic zebrafish larva mono-associated with a fluorescently labeled strain of bacteria.
Figure: This image depicts an axenic zebrafish larva mono-associated with a fluorescently labeled strain of bacteria.
mTox Microbiome Team: Sebastian Gutsfeld, Nicole Schweiger, Chloe Wray, Siraz Kader, Tamara Tal
Papers
DOI: 10.1093/toxsci/kfz166
DOI: 10.1186/s42523-020-00069-x
Large scale data
mTox generates a vast amount of automated bright field and fluorescence imaging data, automated behavior datas, and underlying videos. We are working towards making our data Findable, Accessible, Interoperable, and Reusable (FAIR). We apply and integrate open source computational tools for reproducible effect assessment such as R or Python for our workflows in GALAXY KNIME or SHINY. Our imaging and effect data are archived in databases such as OMERO or INTOB. Our software tools are made available publicly via gitlab or github repositories.
mTox Data Team: Sebastian Gutsfeld, Elena Nicolay, Stefan Scholz, Tamara Tal
Effects of endocrine disrupting chemicals (EDCs) on intestinal inflammation and enteric nervous system development
To bridge chemical exposure and behavior effects, another interest of the group is to develop high-content imaging approaches to quantify cellular and sub-cellular effects of chemicals and mixtures. In the ENDOMIX project, a combination of transgenic zebrafish lines is used to understand how commonly co-occurring EDC chemicals might cause inflammation of the intestinal tract and/or alter enteric nervous system development. Funded by ENDOMIX.
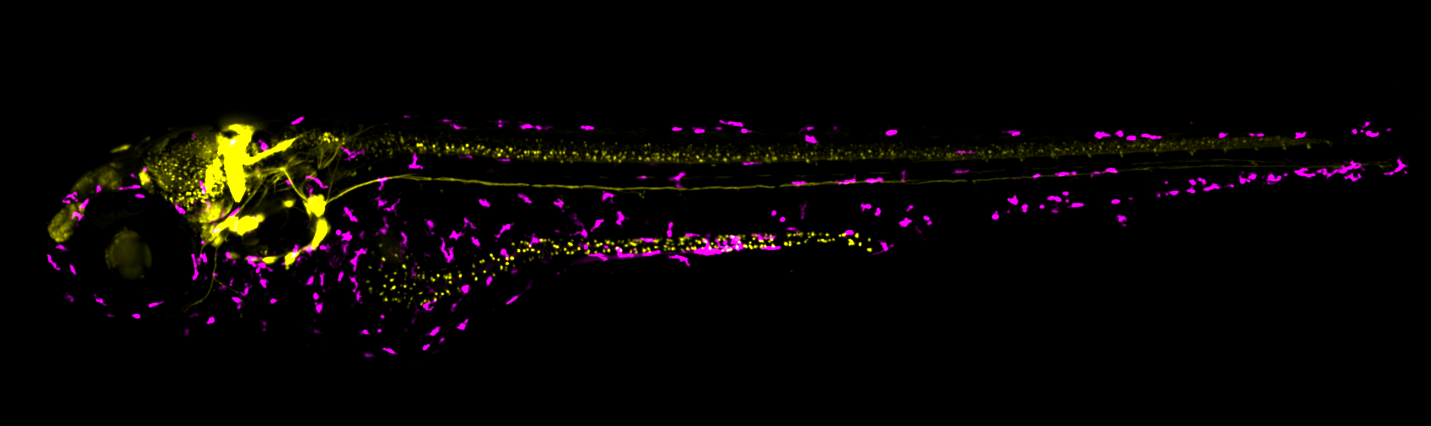 Figure: The maximum projection shows a five-day-old double-transgenic zebrafish larva (Tg(neurod:GFP) x Tg(mpeg1:mCherry)). Neurons, including the ones located in the intestine, are labelled in yellow (neurod), and macrophages in magenta (mpeg1), modelling their interplay under environmental chemical exposure.
Figure: The maximum projection shows a five-day-old double-transgenic zebrafish larva (Tg(neurod:GFP) x Tg(mpeg1:mCherry)). Neurons, including the ones located in the intestine, are labelled in yellow (neurod), and macrophages in magenta (mpeg1), modelling their interplay under environmental chemical exposure.
EDC Team: Elena Nicolay, Tamara Tal
mTOX Group Leader
 Prof. Dr. Tamara Tal leads the Mechanistic Toxicology Group at the Helmholtz Centre for Environmental Research – UFZ. She also holds a Professorship in Integrated Systems Toxicology in the Medical Faculty at University Leipzig. Prior to joining the UFZ in 2019, Tamara was a Principal Investigator at the United States Environmental Protection Agency in the Office of Research and Development. Tamara completed postdoctoral fellowships in the labs of Dr. Robyn Tanguay (Oregon State University) and Dr. Stephanie Padilla (EPA) and earned a doctorate in toxicology from the University of North Carolina at Chapel Hill under the mentorship of Dr. James Samet. Tamara leads New Approach Method development for developmental and adult neurotoxicity endpoints in the European Partnership for the Assessment of Risks from Chemicals (PARC) and participates in the PANDORA and ENDOMIX consortia.
Prof. Dr. Tamara Tal leads the Mechanistic Toxicology Group at the Helmholtz Centre for Environmental Research – UFZ. She also holds a Professorship in Integrated Systems Toxicology in the Medical Faculty at University Leipzig. Prior to joining the UFZ in 2019, Tamara was a Principal Investigator at the United States Environmental Protection Agency in the Office of Research and Development. Tamara completed postdoctoral fellowships in the labs of Dr. Robyn Tanguay (Oregon State University) and Dr. Stephanie Padilla (EPA) and earned a doctorate in toxicology from the University of North Carolina at Chapel Hill under the mentorship of Dr. James Samet. Tamara leads New Approach Method development for developmental and adult neurotoxicity endpoints in the European Partnership for the Assessment of Risks from Chemicals (PARC) and participates in the PANDORA and ENDOMIX consortia.
mTox Group members

Team members
Lead
Prof. Dr. Tamara Tal
Senior scientist
2019 - present
Email
Dr Stefan Scholz
Senior scientist
2002 - present
Email
Dr David Leuthold
Postdoctoral scientist
2019 - present
Email
Camila Zanini
Researcher
2022 - present
E-Mail
Nadia Herold
PhD Student
2022 - present
E-Mail
Alumni
Julia Nöth, Doctoral student 2020-2022. Next Position: Heraeus, DE.
Gabriel de Macedo, DAAD Visiting PhD Fellow, October 2022 - February 2023.
Ifeoluwa Omoyeni, Erasmus Master’s Student, February-July 2022. Next position: Associate Toxicologist, Broughton Life Sciences, UK
Dr. Luísa Becker Bertotto, US EPA ORISE Postdoctoral Fellow, 2018-2019. Next position: Postdoctoral Fellow, Scripps Research Institute.
Shaza Gaballah, US EPA ORISE Post-Baccalaureate Trainee, 2017-2019. Next position: PhD student at Duke University, Stapleton Lab.
Allison Kvasnicka, US EPA Undergraduate Research Volunteer (Summer 2017) and ORISE trainee (Summer 2018). Next position: Research Specialist, BioAgilytix.
Dr. Tara Catron, US EPA ORISE Postdoctoral Fellow. Next position: Ecotoxicologist, BASF.
Drake Phelps, US EPA ORISE Post-Baccalaureate Trainee, 2016-2017. Next position: PhD Student NCSU Neuroimmunology Program, Yoder Lab.
Publications
Index:
- 2026 (2)
- 2025 (32)
- 2024 (9)
- 2023 (11)
- 2022 (12)
- 2021 (13)
- 2020 (10)
- 2019 (8)
- 2018 (9)
- 2017 (4)
- 2016 (6)
- 2015 (8)
You could use our publication index for further requests.
2026 (2)
- Escher, B.I., Blackwell, B.R., Cavallin, J., Dann, J.P., Jahnke, A., Jenson, C., Jensen, K., Kahl, M., Krauss, M., König, M., Neale, P.A., Scholz, S., Villeneuve, D., Ankley, G.T. (2026):
In vitro bioassays for quantifying mixture effects of organic micropollutants extracted from caged fish, water, and sediment
Environ. Toxicol. Chem. 45 (1), 137 - 151 10.1093/etojnl/vgaf255 - Herold, N.K., Sørensen, L., Creese, M.E., Nahrgang, J., Schweiger, N., Tal, T. (2026):
Identification of neuroactive chemicals in crude oil-derived water-accommodated fractions
Environ. Sci. Technol. 60 (1), 181 - 195 10.1021/acs.est.5c06859
2025 (32)
- Ayuk, H.S., Pierzchalski, A., Tal, T., Myhre, O., Lindeman, B., Smith, N.M., Stojanovska, V., Zenclussen, A.C. (2025):
Evaluating PFAS-Induced modulation of peripheral blood mononuclear cells (PBMCs) immune response to SARS-CoV-2 spike in COVID-19 Vaccinees
Environ. Int. 198 , art. 109409 10.1016/j.envint.2025.109409 - Celardo, I., Aschner, M., Ashton, R.S., Carstens, K.E., Cediel-Ulloa, A., Cöllen, E., Crofton, K.M., Debad, S.J., Dreser, N., Fitzpatrick, S., Fritsche, E., Gutsfeld, S., Hardy, B., Hartung, T., Hessel, E., Heusinkveld, H., Hogberg, H.T., Hsieh, J.-H., Kanda, Y., Knight, G.T., Knudsen, T., Koch, K., Kuchovska, E., Mangas, I., Marty, M.S., Melching-Kollmuss, S., Müller, I., Müller, P., Myhre, O., Paparella, M., Pitzer, E., Bal-Price, A., Sachana, M., Schlüppmann, K., Shafer, T.J., Schäfer, J., Smirnova, L., Tal, T., Tanaskov, Y., Tangianu, S., Testa, G., Ückert, A.-K., Whelan, M., Leist, M. (2025):
Developmental neurotoxicity (DNT): A call for implementation of new approach methodologies for regulatory purposes: Summary of the 5th International Conference on DNT Testing
ALTEX-Altern. Anim. Exp. 42 (2), 323 - 349 10.14573/altex.2503191 - Dann, J.P., Ankley, G.T., Blackwell, B.R., Escher, B.I., Jahnke, A., Jensen, K.M., Jenson, C., Krauss, M., Scholz, S., Wernicke, T., Brack, W. (2025):
Current emission vs. legacy organic pollutants: Assessing the extent to which the eco-exposome of caged fish reflects external exposure
Environ. Pollut. 383 , art. 126808 10.1016/j.envpol.2025.126808 - Glaberman, S., Frey, H.C., Tal, T. (2025):
Dismantling EPA’s research office jeopardizes environmental safety, public health, and US competitiveness
Proc. Natl. Acad. Sci. U.S.A. 122 (24), e2508060122 10.1073/pnas.2508060122 - Gómez-Olarte, S., Kretschmer, T., Zantop, S., Tal, T., Myhre, O., Stojanovska, V., Meyer, N., Zenclussen, A.C. (2025):
PFAS mixture effects on single-cell CyTOF-based profiling of human primary immune cells: transitioning to new approach methodologies
Toxicol. Lett. 411 (Supplement), S184 - S185 10.1016/j.toxlet.2025.07.444 - Gutsfeld, S., Wray, C., Schweiger, N., Röhrig, A., Paschke, H., Fu, Q., Kasmanas, J.C., Kader, S., Nunes da Rocha, U., Tal, T. (2025):
The zebrafish microbiome has the capacity to bioactivate PFOS precursor compounds
Toxicol. Lett. 411 (Supplement), S176 10.1016/j.toxlet.2025.07.425 - Hayot, G., Lloyd, G.R., Diwan, G.D., Keith, N., Smoot, S.R., Cramer von Clausbruch, C.A., Kaufman, T.C., Barnard, M., Tindall, A.J., Glaholt, S.P., Massei, R., Martínez, R., Strähle, U., Orsini, L., Russell, R.B., Tennessen, J.M., Scholz, S., Shaw, J.R., Freedman, J.H., Colbourne, J.K., Weiss, C., Dickmeis, T. (2025):
Alternative vertebrate and invertebrate model organisms show similar sensitivity as rodents to a diverse set of chemicals
Environ. Sci. Technol. 59 (48), 25634 - 25648 10.1021/acs.est.5c10177 - Herold, N.K., Gutsfeld, S., Leuthold, D., Wray, C., Spath, J., Tal, T. (2025):
Multi-behavioral fingerprints can identify potential modes of action for neuroactive environmental chemicals
NeuroToxicology 108 , 377 - 399 10.1016/j.neuro.2025.05.001 - Herold, N.K., Sørensen, L., Creese, M.E., Nahrgang, J., Schweiger, N., Scholz, S., Tal, T. (2025):
Neuroactive behavioral fingerprinting of crude oil-derived water accommodated fractions in larval zebrafish using a new approach method
Toxicol. Lett. 411 (Supplement), S119 10.1016/j.toxlet.2025.07.302 - Herold, N.K., Sørensen, L., Creese, M.E., Nahrgang, J., Schweiger, N., Tal, T. (2025):
Chemical and Behavioral Fingerprinting Data for the Identification of Neuroactive Compounds in Crude Oil-Derived Water Accommodated Fractions [V3]
Mendeley Data 10.17632/8hmr85pjw7.3 - Küster, E., Addo, G.G., Aulhorn, S., Kühnel, D. (2025):
Miniaturisation of the Daphnia magna immobilisation assay for the reliable testing of low volume samples
UCL Open Environ. 7 (1), art. 3037 10.14324/111.444/ucloe.3037 - Leuthold, D., Herold, N.K., Nerlich, J., Bartmann, K., Scharkin, I., Hallermann, S.J., Schweiger, N., Fritsche, E., Tal, T. (2025):
Multi-behavioral phenotyping in early-life-stage zebrafish for identifying disruptors of non-associative learning
Environ. Health Perspect. 10.1289/EHP16568 - Leuthold, D., Herold, N., Nerlich, J., Bartmann, K., Scharkin, I., Hallermann, S.J., Schweiger, N., Fritsche, E., Tal, T. (2025):
Data used in Leuthold et al. "Multi-behavioral phenotyping in early-life-stage zebrafish for identifying disruptors of non-associative learning"
figshare 10.6084/m9.figshare.26819890 - Martínez, R., González-Sánchez, J.C., Sampani, S.I., Scholz, S., Escher, B.I., Henneberger, L., Huchthausen, J., Whelan, M., Dickmeis, T., Weiss, C., Colbourne, J.K., Freedman, J.H. (2025):
The PrecisionTox chemical library: creation of a chemical collection to discover evolutionary conserved biomolecular signatures of toxicity
Toxicol. Sci. 208 (2), 317 - 329 10.1093/toxsci/kfaf126 - Massei, R., Busch, W., Serrano-Solano, B., Bernt, M., Scholz, S., Nicolay, E.K., Bohring, H., Bumberger, J. (2025):
High-content screening (HCS) workflows for FAIR image data management with OMERO
Sci. Rep. 15 , art. 16236 10.1038/s41598-025-00720-0 - Michaelis, P., Klüver, N., Aulhorn, S., Bohring, H., Bumberger, J., Haase, K., Kuhnert, T., Küster, E., Krüger, J., Luckenbach, T., Massei, R., Nerlich, L., Petruschke, S., Schnicke, T., Schnurpel, A., Scholz, S., Schweiger, N., Sielaff, D., Busch, W. (2025):
Leveraging zebrafish embryo phenotypic observations to advance data-driven analyses in toxicology
Environ. Sci. Technol. 59 (9), 4304 - 4317 10.1021/acs.est.4c11757 - Nicolay, E.K., Massei, R., Trofimova, D., Haase, R., Isensee, F., Tal, T. (2025):
Establishing a high-content imaging workflow to investigate the effect of environmental chemicals on macrophages and enteric neurons in zebrafish larvae
Neurogastroenterol. Motil. 37 (S2), e70126 - NGS21070-82 10.1111/nmo.70126 - Nöth, J., Michaelis, P., Schüler, L., Scholz, S., Krüger, J., Haake, V., Busch, W. (2025):
Correction to: Dynamics in zebrafish development define transcriptomic specificity after angiogenesis inhibitor exposure
Arch. Toxicol. 99 (4), 1579 10.1007/s00204-025-03998-1 - Nöth, J., Michaelis, P., Schüler, L., Scholz, S., Krüger, J., Haake, V., Busch, W. (2025):
Dynamics in zebrafish development define transcriptomic specificity after angiogenesis inhibitor exposure
Arch. Toxicol. 99 (4), 1561 - 1578 10.1007/s00204-024-03944-7 - Nyffeler, J., Tal, T., Schildknecht, S., Viviani, B., Tanja, B., Fu, Q., Owen, R., Krishnakumar, A.E.V., Mangas, I., Terron, A. (2025):
Towards a defined approach to assess pesticides for their potential to cause Parkinsonian neurodegeneration
Toxicol. Lett. 411 (Supplement), S397 - S398 10.1016/j.toxlet.2025.07.916 - Owen, R., de Macedo, G., Nerlich, J., Scharkin, I., Bartmann, K., Döbler, J., Engelmann, B., Rolle-Kampczyk, U.E., Leuthold, D., Gutsfeld, S., Schweiger, N., Tal, T. (2025):
Perfluorooctanesulfonic acid (PFOS) antagonizes gamma-aminobutyric acid (GABA) receptors in larval zebrafish and mammalian models
Toxicol. Sci. 207 (2), 449 - 466 10.1093/toxsci/kfaf101 - Owen, R., de Macedo, G., Nerlich, J., Scharkin, I., Bartmann, K., Döbler, J., Engelmann, B., Rolle-Kampczyk, U., Leuthold, D., Gutsfeld, S., Schweiger, N., Tal, T. (2025):
Dataset for PFOS antagonism of GABA receptors in vertebrate models
Zenodo 10.5281/zenodo.15394336 - Owen, R., Herzke, D., Haug, L.S., Myhre, O., Nerlich, J., Scharkin, I., Bartmann, K., Tal, T. (2025):
Exposure to a human-relevant PFAS mixture causes behavioral effects in larval zebrafish: A focus on chemical drivers, phenotypes, and underlying mechanisms
Toxicol. Lett. 411 (Supplement), S56 - S57 10.1016/j.toxlet.2025.07.165 - Raab, J., Gutsfeld, S., Tal, T. (2025):
NeuroBEAT: A comprehensive and flexible behavior analysis tool for neurotoxicity testing in larval zebrafish
Toxicol. Lett. 411 (Supplement), S109 10.1016/j.toxlet.2025.07.282 - Schmidt, M., Aulhorn, S., Latif, A.A., Krauss, M., Schmitt-Jansen, M., Breite, D., Küster, E., Schulze, A. (2025):
Photocatalytic membrane treatment of antibiotics: combined chemical and toxicological evaluation of effectiveness
Front. Env. Sci. Eng. 19 (12), art. 163 10.1007/s11783-025-2083-7 - Scholz, S., Zanini, C., Koblitz, A.-K., Möller, T., Aslam, M.A., Ajugwo, G.C., Chukwu, E., Colbourne, J., Kader, S., Fu, Q., Grasse, N., Reemtsma, T., Massei, R. (2025):
Grouping and assessment of chemicals for hazard and risk assessment by high content analysis using the zebrafish embryos as an alternative non-sentient animal model
Toxicol. Lett. 411 (Supplement), S399 10.1016/j.toxlet.2025.07.919 - Tal, T. (2025):
The microbiome modifies neurobehavior in zebrafish exposed to Aryl Hydrocarbon Receptor (AHR) modulators
Toxicol. Lett. 411 (Supplement), S45 - S46 10.1016/j.toxlet.2025.07.138 - Tanabe, S., Burgdorf, T., Choi, J., Delrue, N., Edwards, S.W., Filipovska, J., FitzGerald, R., Halappanavar, S., Hench, V.K., Karschnik, T., LaLone, C., Landesmann, B., La Rocca, C., Luijten, M., Meek, B., O’Brien, J.M., Perkins, E.J., Perkins, S., Scholz, S., Song, Y., Tcheremenskaia, O., Thomas, R., Tollefsen, K.E., Villeneuve, D.L., Viviani, B., Whelan, M., Wittwehr, C., Yauk, C. (2025):
Adverse Outcome Pathway (AOP) Coaching Program—how it functions and contributes to a more harmonized approach to AOP development and construction of AOP networks with regulatory utility
Environ. Toxicol. Chem. 44 (10), 2725 - 2732 10.1093/etojnl/vgaf173 - Uthoff, C., Herold, N., Alkassab, A.T., Engelmann, B., Rolle-Kampczyk, U., Pistorius, J., Schweiger, N., Finckh, S., Krauss, M., Thum, A.S., Jehmlich, N., Tal, T., von Bergen, M. (2025):
Cross-taxa sublethal impacts of plant protection products on honeybee in-hive and zebrafish swimming behaviours at environmentally relevant concentrations
Environ. Int. 203 , art. 109750 10.1016/j.envint.2025.109750 - Uthoff, C., Herold, N., Alkassab, A.T., Engelmann, B., Rolle-Kampczyk, U., Pistorius, J., Schweiger, N., Finckh, S., Krauss, M., Thum, A.S., Jehmlich, N., Tal, T., von Bergen, M. (2025):
Dataset for 'Cross-taxa sublethal impacts of plant protection products on honeybee in-hive and zebrafish swimming behaviours at environmentally relevant concentrations'
Zenodo 10.5281/zenodo.16983482 - Wray, C., Kader, S., Escher, B.I., Henneberger, L., Krauss, M., Schweiger, N., Owen, R., Tyler, C.R., Tal, T. (2025):
Chemical-microbiome interactions in larval zebrafish: Unraveling the interactive effects of microbiome induced biotransformation of PAHs and host behavior
Toxicol. Lett. 411 (Supplement), S174 10.1016/j.toxlet.2025.07.421 - Zenclussen, A.C., Belmar Erilkin, V., Böhmert, L., Borilova Linhartova, P., Braeuning, A., Braun, G., Chevrier, C., Duijts, L., Escher, B.I., Felix, J., Gómez-Olarte, S., Guxens, M., Herberth, G., Hilscherova, K., Klanova, J., Kohl, Y., Krischak, K., Lagadic-Gossmann, D., Langouët, S., Llop, S., Lopez-Espinosa, M.-J., Maitre, L., Martin-Chouly, C., Meyer, N., Ouidir, M., Pham, T.A.M., Philippat, C., Pieters, R., Pinel-Marie, M.-L., Podechard, N., Polte, T., Price, E., Robinson, O., Schubert, K., Schumacher, A., Stojanovska, V., Tal, T., Vineis, P., van Vorstenbosch, R., Vermeulen, R., Warembourg, C. (2025):
The ENDOMIX project: an interdisciplinary approach to understanding how real-life chemical mixtures target the immune system to trigger disease [version 2]
Open Research Europe 4 , art. 271 10.12688/openreseurope.19088.2
2024 (9)
- Escher, B.I., Ahlheim, J., Böhme, A., Borchardt, D., Brack, W., Braun, G., Colbourne, J.K., Dann, J.P., Gessner, J., Jahnke, A., König, M., Klüver, N., Krauss, M., Lee, J., Li, X., Lips, S., Orsini, L., Rinke, K., Schmitt-Jansen, M., Scholz, S., Schulze, T., Spahr, S., Ulrich, N., Weitere, M., Varga, E. (2024):
Mixtures of organic micropollutants exacerbated in vitro neurotoxicity of prymnesins and contributed to aquatic toxicity during a toxic algal bloom
Nat. Water 2 (9), 889 - 898 10.1038/s44221-024-00297-4 - Grasse, N., Massei, R., Seiwert, B., Scholz, S., Escher, B.I., Reemtsma, T., Fu, Q. (2024):
Impact of biotransformation on internal concentrations and specificity classification of organic chemicals in the zebrafish embryo (Danio rerio)
Environ. Sci. Technol. 58 (40), 17898 - 17907 10.1021/acs.est.4c04156 - Gutsfeld, S., Wehmas, L., Omoyeni, I.E., Schweiger, N., Leuthold, D., Michaelis, P., Howey, X.M., Gaballah, S., Herold, N., Vogs, C., Wood, C., Bertotto, L., Wu, G.-M., Klüver, N., Busch, W., Scholz, S., Schor, J., Tal, T. (2024):
Investigation of peroxisome proliferator-activated receptor genes as requirements for visual startle response hyperactivity in larval zebrafish exposed to structurally similar per- and polyfluoroalkyl substances (PFAS)
Environ. Health Perspect. 132 (7), art. 077007 10.1289/EHP13667 - Hayot, G., Marcato, D., Cramer von Clausbruch, C.A., Pace, G., Strähle, U., Colbourne, J.K., Pylatiuk, C., Peravali, R., Weiss, C., Scholz, S., Dickmeis, T. (2024):
Evaluating toxicity of chemicals using a zebrafish vibration startle response screening system
J. Vis. Exp. 2024 (203), e66153 10.3791/66153 - Hayot, G., Massei, R., Lloyd, G., Keith, N., Diwan, G., Martinez Lopez, R., Barnard, M., Cramer von Clausbruch, C.A., Grasse, N., Smoot, S., Escher, B., Tennessen, J., Tindall, A., Oliver, B., Shaw, J., Scholz, S., Freedman, J., Strähle, U., Colbourne, J., Weiss, C., Dickmeis, T. (2024):
Systematic acquisition of toxicity data in non- sentient models across animal phylogeny: implications for read- across and estimation of toxicity in humans
Naunyn-Schmiedebergs Arch. Pharmacol. 397 (Suppl. 1), S17 - S18-69 10.1007/s00210-024-02974-3 - Herold, N.K. (2024):
Multi-behavioral fingerprints can identify the mode of action for neuroactive environmental chemicals _ VAMR2
Mendeley Data 10.17632/97f3yffwz9.1 - Nöth, J., Busch, W., Tal, T., Lai, C., Ambekar, A., Kießling, T.R., Scholz, S. (2024):
Analysis of vascular disruption in zebrafish embryos as an endpoint to predict developmental toxicity
Arch. Toxicol. 98 (2), 537 - 549 10.1007/s00204-023-03633-x - Tal, T., Myhre, O., Fritsche, E., Rüegg, J., Craenen, K., Aiello-Holden, K., Agrillo, C., Babin, P.J., Escher, B.I., Dirven, H., Hellsten, K., Dolva, K., Hessel, E., Heusinkveld, H.J., Hadzhiev, Y., Hurem, S., Jagiello, K., Judzinska, B., Klüver, N., Knoll-Gellida, A., Kühne, B.A., Leist, M., Lislien, M., Lyche, J.L., Müller, F., Colbourne, J.K., Neuhaus, W., Pallocca, G., Seeger, B., Scharkin, I., Scholz, S., Spjuth, O., Torres-Ruiz, M., Bartmann, K. (2024):
New approach methods to assess developmental and adult neurotoxicity for regulatory use: a PARC work package 5 project
Front. Toxicol. 6 , art. 1359507 10.3389/ftox.2024.1359507 - Wilhelmi, P., Haake, V., Zickgraf, F.M., Giri, V., Ternes, P., Driemert, P., Nöth, J., Scholz, S., Barenys, M., Flick, B., Birk, B., Kamp, H., Landsiedel, R., Funk-Weyer, D. (2024):
Molecular signatures of angiogenesis inhibitors: a single-embryo untargeted metabolomics approach in zebrafish
Arch. Toxicol. 98 (3), 943 - 956 10.1007/s00204-023-03655-5
2023 (11)
- Avila Santos, A.P., Kabiru Nata’ala, M., Kasmanas, J.C., Bartholomäus, A., Keller-Costa, T., Jurburg, S.D., Tal, T., Camarinha-Silva, A., Saraiva, J.P., de Carvalho, A.C.P.L.F., Stadler, P.F., Sipoli Sanches, D., Nunes da Rocha, U. (2023):
The AnimalAssociatedMetagenomeDB reveals a bias towards livestock and developed countries and blind spots in functional-potential studies of animal-associated microbiomes
Animal Microbiome 5 , art. 48 10.1186/s42523-023-00267-3 - Bajard, L., Adamovsky, O., Audouze, K., Baken, K., Barouki, R., Beltman, J.B., Beronius, A., Bonefeld-Jørgensen, E.C., Cano-Sancho, G., de Baat, M.L., Di Tillio, F., Fernández, M.F., FitzGerald, R.E., Gundacker, C., Hernández, A.F., Hilscherova, K., Karakitsios, S., Kuchovska, E., Long, M., Luijten, M., Majid, S., Marx-Stoelting, P., Mustieles, V., Negi, C.K., Sarigiannis, D., Scholz, S., Sovadinova, I., Stierum, R., Tanabe, S., Tollefsen, K.E., van den Brand, A.D., Vogs, C., Wielsøe, M., Wittwehr, C., Blaha, L. (2023):
Application of AOPs to assist regulatory assessment of chemical risks – Case studies, needs and recommendations
Environ. Res. 217 , art. 114650 10.1016/j.envres.2022.114650 - Bashirova, N., Poppitz, D., Klüver, N., Scholz, S., Matysik, J., Alia, A. (2023):
A mechanistic understanding of the effects of polyethylene terephthalate nanoplastics in the zebrafish (Danio rerio) embryo
Sci. Rep. 13 , art. 1891 10.1038/s41598-023-28712-y - Colbourne, J.K., Andrews, E., Barnard, M., Čavoški, A., Chaturvedi, A., Epps, D.J.T., Holden, L., Jones, M.R., Li, X., Mueller, F., Ormanin-Lewandowska, A., Orsini, L., Roberts, R., Weber, R.J.M., Zhou, J., Apic, G., Ignasiak, T., Jankovic, K., Krsmanovic, T., Leoni, B., Asole, G., Guigó, R., Marangio, P., Palumbo, E., Perez-Lluch, S., Wucher, V., Vlot, A.H., Anholt, R., Mackay, T., Escher, B.I., Grasse, N., Huchthausen, J., Massei, R., Reemtsma, T., Scholz, S., Schüürmann, G., Bondesson, M., Cherbas, P., et al. (2023):
The Precision Toxicology initiative
Toxicol. Lett. 383 , 33 - 42 10.1016/j.toxlet.2023.05.004 - Cramer von Clausbruch, C.A., Weiss, C., Dickmeis, T., Peravali, R., Strähle, U., Colbourne, J., Hayot, G., Scholz, S., Martinez Lopez, R. (2023):
Quantitative and qualitative assessment of cytotoxicity by automated fluorescence microscopy in human hepatocytes
Naunyn-Schmiedebergs Arch. Pharmacol. 396 (Suppl. 1), S61 - S62 10.1007/s00210-023-02397-6 - De Castelbajac, T., Aiello, K., Arenas, C.G., Svingen, T., Ramhøj, L., Zalko, D., Barouki, R., Vanhaecke, T., Rogiers, V., Audebert, M., Oelgeschlaeger, M., Braeuning, A., Blanc, E., Tal, T., Rüegg, J., Fritsche, E., Marx-Stoelting, P., Rivière, G. (2023):
Innovative tools and methods for toxicity testing within PARC work package 5 on hazard assessment
Front. Toxicol. 5 , art. 1216369 10.3389/ftox.2023.1216369 - Escher, B.I., Altenburger, R., Blüher, M., Colbourne, J.K., Ebinghaus, R., Fantke, P., Hein, M., Köck, W., Kümmerer, K., Leipold, S., Li, X., Scheringer, M., Scholz, S., Schloter, M., Schweizer, P.-J., Tal, T., Tetko, I., Traidl-Hoffmann, C., Wick, L.Y., Fenner, K. (2023):
Modernizing persistence–bioaccumulation–toxicity (PBT) assessment with high throughput animal-free methods
Arch. Toxicol. 97 (5), 1267 - 1283 10.1007/s00204-023-03485-5 - Grasse, N., Seiwert, B., Massei, R., Scholz, S., Fu, Q., Reemtsma, T. (2023):
Uptake and biotransformation of the tire rubber-derived contaminants 6-PPD and 6-PPD quinone in the zebrafish embryo (Danio rerio)
Environ. Sci. Technol. 57 (41), 15598 - 15607 10.1021/acs.est.3c02819 - Hayot, G., von Clausbruch, C., Martinez Lopez, R., Scholz, S., Colbourne, J., Strähle, U., Weiss, C., Peravali, R., Dickmeis, T. (2023):
Phenotypic anchoring for OMICS-guided assessment of liver toxicity in zebrafish
Naunyn-Schmiedebergs Arch. Pharmacol. 396 (Suppl. 1), S61 - S61 10.1007/s00210-023-02397-6 - Scholz, S. (2023):
sscholz-UFZ/EmbryoMotion: EmbryoMotion Version 1.1. - a software to analyse spontaneous tail contraction and photomotor response of zebrafish embryos
Version: 1.1 Zenodo 10.5281/zenodo.8362019 - Wilhelmi, P., Giri, V., Zickgraf, F.M., Haake, V., Henkes, S., Driemert, P., Michaelis, P., Busch, W., Scholz, S., Flick, B., Barenys, M., Birk, B., Kamp, H., Landsiedel, R., Funk-Weyer, D. (2023):
A metabolomics approach to reveal the mechanism of developmental toxicity in zebrafish embryos exposed to 6-propyl-2-thiouracil
Chem.-Biol. Interact. 382 , art. 110565 10.1016/j.cbi.2023.110565
2022 (12)
- Halbach, K., Aulhorn, S., Lechtenfeld, O.J., Lecluse, M., Leippe, S., Reemtsma, T., Seiwert, B., Wagner, S., König, J., Luckenbach, T. (2022):
Zebrafish Oatp1d1 acts as a cellular efflux transporter of the anionic herbicide bromoxynil
Chem. Res. Toxicol. 35 (2), 315 - 325 10.1021/acs.chemrestox.1c00371 - Lee, J., Escher, B.I., Scholz, S., Schlichting, R. (2022):
Inhibition of neurite outgrowth and enhanced effects compared to baseline toxicity in SH-SY5Y cells
Arch. Toxicol. 96 (4), 1039 - 1053 10.1007/s00204-022-03237-x - Lee, J., Huchthausen, J., Schlichting, R., Scholz, S., Henneberger, L., Escher, B.I. (2022):
Validation of an SH-SY5Y cell-based acetylcholinesterase inhibition assay for water quality assessment
Environ. Toxicol. Chem. 41 (12), 3046 - 3057 10.1002/etc.5490 - Lee, J., Schlichting, R., König, M., Scholz, S., Krauss, M., Escher, B.I. (2022):
Monitoring mixture effects of neurotoxicants in surface water and wastewater treatment plant effluents with neurite outgrowth inhibition in SH-SY5Y cells
ACS Environ. Au 2 (6), 523 - 535 10.1021/acsenvironau.2c00026 - Nöth, J., Michaelis, P., Busch, W., Scholz, S. (2022):
Analysis vascular disruptors in zebrafish embryos as an endpoint to predict developmental toxicity
Toxicol. Lett. 368 (Suppl.), S34 - S35 10.1016/j.toxlet.2022.07.111 - Ortmann, J., Altenburger, R., Scholz, S., Luckenbach, T. (2022):
Photomotor response data analysis approach to assess chemical neurotoxicity with the zebrafish embryo
ALTEX-Altern. Anim. Exp. 39 (1), 82 - 94 10.14573/altex.2004021 - Paini, A., Campia, I., Cronin, M.T.D., Asturiol, D., Ceriani, L., Exner, T.E., Gao, W., Gomes, C., Kruisselbrink, J., Martens, M., Meek, M.E.B., Pamies, D., Pletz, J., Scholz, S., Schüttler, A., Spînu, N., Villeneuve, D.L., Wittwehr, C., Worth, A., Luijten, M. (2022):
Towards a qAOP framework for predictive toxicology - Linking data to decisions
Comput. Toxicol. 21 , art. 100195 10.1016/j.comtox.2021.100195 - Scholz, S., Brack, W., Escher, B.I., Hackermüller, J., Liess, M., von Bergen, M., Wick, L.Y., Zenclussen, A.C., Altenburger, R. (2022):
The EU chemicals strategy for sustainability: an opportunity to develop new approaches for hazard and risk assessment
Arch. Toxicol. 96 (8), 2381 - 2386 10.1007/s00204-022-03313-2 - Scholz, S., Nichols, J.W., Escher, B.I., Ankley, G.T., Altenburger, R., Blackwell, B., Brack, W., Burkhard, L., Collette, T.W., Doering, J.A., Ekman, D., Fay, K., Fischer, F., Hackermüller, J., Hoffman, J.C., Lai, C., Leuthold, D., Martinovic-Weigelt, D., Reemtsma, T., Pollesch, N., Schroeder, A., Schüürmann, G., von Bergen, M. (2022):
The eco‐exposome concept: Supporting an integrated assessment of mixtures of environmental chemicals
Environ. Toxicol. Chem. 41 (1), 30 - 45 10.1002/etc.5242 - Spînu, N., Cronin, M.T.D., Lao, J., Bal-Price, A., Campia, I., Enoch, S.J., Madden, J.C., Lagares, L.M., Novič, M., Pamies, D., Scholz, S., Villeneuve, D.L., Worth, A.P. (2022):
Probabilistic modelling of developmental neurotoxicity based on a simplified adverse outcome pathway network
Comput. Toxicol. 21 , art. 100206 10.1016/j.comtox.2021.100206 - Teixidó, E., Kieβling, T.R., Klüver, N., Scholz, S. (2022):
Grouping of chemicals into mode of action classes by automated effect pattern analysis using the zebrafish embryo toxicity test
Arch. Toxicol. 96 (5), 1353 - 1369 10.1007/s00204-022-03253-x - Wilhelmi, P., Giri, V., Henkes, S., Walk, T., Haake, V., Scholz, S., Busch, W., Barenys, M., Zickgraf, F., Landsiedel, R., Funk-Weyer, D., Birk, B., Flick, B. (2022):
A targeted metabolomics approach for unraveling different modes of embryotoxicity in zebrafish
Toxicol. Lett. 368 (Suppl.), S252 - S252 10.1016/j.toxlet.2022.07.671
2021 (13)
- Ambekar, A., Poschen, C., Lai, C., Scholz, S., Teixidó, E. (2021):
Analyzing morphological changes in zebrafish embryos exposed to toxic chemicals
2021 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Houston, TX, USA, 9-12 December 2021
Institute of Electrical and Electronics Engineers (IEEE), New York, NY, p. 1711 - 1718 10.1109/BIBM52615.2021.9669338 - Bieczynski, F., Burkhardt-Medicke, K., Luquet, C.M., Scholz, S., Luckenbach, T. (2021):
Chemical effects on dye efflux activity in live zebrafish embryos and on zebrafish Abcb4 ATPase activity
FEBS Lett. 595 (6), 828 - 843 10.1002/1873-3468.14015 - Huhn, S., Escher, B.I., Krauss, M., Scholz, S., Hackermüller, J., Altenburger, R. (2021):
Unravelling the chemical exposome in cohort studies: routes explored and steps to become comprehensive
Environ. Sci. Eur. 33 , art. 17 10.1186/s12302-020-00444-0 - Kasmanas, J.C., Bartholomäus, A., Borim Corrêa, F., Tal, T., Jehmlich, N., Herberth, G., von Bergen, M., Stadler, P.F., de Carvalho, A.C.P.L.F., Nunes da Rocha, U. (2021):
HumanMetagenomeDB: a public repository of curated and standardized metadata for human metagenomes
Nucleic Acids Res. 49 (D1), D743 - D750 10.1093/nar/gkaa1031 - Lee, J., Braun, G., Henneberger, L., König, M., Schlichting, R., Scholz, S., Escher, B.I. (2021):
Critical membrane concentration and mass-balance model to identify baseline cytotoxicity of hydrophobic and ionizable organic chemicals in mammalian cell lines
Chem. Res. Toxicol. 34 (9), 2100 - 2109 10.1021/acs.chemrestox.1c00182 - Ogungbemi, A.O., Massei, R., Altenburger, R., Scholz, S., Küster, E. (2021):
Assessing combined effects for mixtures of similar and dissimilar acting neuroactive substances on zebrafish embryo movement
Toxics 9 (5), art. 104 10.3390/toxics9050104 - Ogungbemi, A.O., Teixido, E., Massei, R., Scholz, S., Küster, E. (2021):
Automated measurement of the spontaneous tail coiling of zebrafish embryos as a sensitive behavior endpoint using a workflow in KNIME
MethodsX 8 , art. 101330 10.1016/j.mex.2021.101330 - Paparella, M., Scholz, S., Belanger, S., Braunbeck, T., Bicherel, P., Connors, K., Faßbender, C., Halder, M., Lillicrap, A., Liska, R., Schirmer, K., Stoddart, G., Thomas, P., Walter-Rohde, S. (2021):
Limitations and uncertainties of acute fish toxicity assessments can be reduced using alternative methods
ALTEX-Altern. Anim. Exp. 38 (1), 20 - 32 10.14573/altex.2006051 - Schüttler, A., Jakobs, G., Fix, J.M., Krauss, M., Krüger, J., Leuthold, D., Altenburger, R., Busch, W. (2021):
Transcriptome-wide prediction and measurement of combined effects induced by chemical mixture exposure in zebrafish embryos
Environ. Health Perspect. 129 (4), art. 047006 10.1289/EHP7773 - Tal, T., Vogs, C. (2021):
Invited perspective: PFAS bioconcentration and biotransformation in early life stage zebrafish and its implications for human health protection
Environ. Health Perspect. 129 (7), art. 071304 10.1289/EHP9625 - Teixidó, E., Klüver, N., Ogungbemi, A.O., Küster, E., Scholz, S. (2021):
Evaluation of neurotoxic effects in zebrafish embryos by automatic measurement of early motor behaviors
In: Llorens, J., Barenys, M. (eds.)
Experimental neurotoxicology methods
Neuromethods 172
Springer Nature, p. 381 - 397 10.1007/978-1-0716-1637-6_17 - Volz, D.C., Cannon, J., Tal, T. (2021):
Introduction to leveraging non-mammalian models for developmental neurotoxicity testing
Neurotoxicol. Teratol. 87 , art. 107001 10.1016/j.ntt.2021.107001 - Weitekamp, C.A., Kvasnicka, A., Keely, S.P., Brinkmann, N.E., Howey, X.M., Gaballah, S., Phelps, D., Catron, T., Zurlinden, T., Wheaton, E., Tal, T. (2021):
Monoassociation with bacterial isolates reveals the role of colonization, community complexity and abundance on locomotor behavior in larval zebrafish
Animal Microbiome 3 , art. 12 10.1186/s42523-020-00069-x
2020 (10)
- Bertotto, L.B., Catron, T.R., Tal, T. (2020):
Exploring interactions between xenobiotics, microbiota, and neurotoxicity in zebrafish
NeuroToxicology 76 , 235 - 244 10.1016/j.neuro.2019.11.008 - Gaballah, S., Swank, A., Sobus, J.R., Howey, X.M., Schmid, J., Catron, T., McCord, J., Hines, E., Strynar, M., Tal, T. (2020):
Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS
Environ. Health Perspect. 128 (4), art. 047005 10.1289/EHP5843 - Halbach, K., Ulrich, N., Goss, K.-U., Seiwert, B., Wagner, S., Scholz, S., Luckenbach, T., Bauer, C., Schweiger, N., Reemtsma, T. (2020):
Yolk sac of zebrafish embryos as backpack for chemicals?
Environ. Sci. Technol. 54 (16), 10159 - 10169 10.1021/acs.est.0c02068 - Larras, F., Billoir, E., Scholz, S., Tarkka, M., Wubet, T., Delignette-Muller, M.-L., Schmitt-Jansen, M. (2020):
A multi-omics concentration-response framework uncovers novel understanding of triclosan effects in the chlorophyte Scenedesmus vacuolatus
J. Hazard. Mater. 397 , art. 122727 10.1016/j.jhazmat.2020.122727 - Leuthold, D. (2020):
Zebrafish locomotor activity as a sensitive effect-based tool for the assessment of environmental chemicals and mixtures
Dissertation, Rheinisch-Westfälische Technische Hochschule (RWTH), Fakultät für Mathematik, Informatik und Naturwissenschaften
PhD Dissertation 04/2020
Helmholtz-Zentrum für Umweltforschung - UFZ, Leipzig, xxiv, 156 pp. - Ogungbemi, A.O., Teixido, E., Massei, R., Scholz, S., Küster, E. (2020):
Optimization of the spontaneous tail coiling test for fast assessment of neurotoxic effects in the zebrafish embryo using an automated workflow in KNIME®
Neurotoxicol. Teratol. 81 , art. 106918 10.1016/j.ntt.2020.106918 - Shatilina, Z., Drozdova, P., Bedulina, D., Rivarola-Duarte, L., Schreiber, S., Otto, C., Jühling, F., Aulhorn, S., Busch, W., Lubyaga, Y., Kondrateva, E., Pobezhimova, T., Jakob, L., Lucassen, M., Sartoris, F.J., Hackermüller, J., Pörtner, H.-O., Stadler, P.F., Luckenbach, T., Timofeyev, M. (2020):
Transcriptome-level effects of the model organic pollutant phenanthrene and its solvent acetone in three amphipod species
Comp. Biochem. Physiol. D-Genomics Proteomics 33 , art. 100630 10.1016/j.cbd.2019.100630 - Tal, T., Yaghoobi, B., Lein, P.J. (2020):
Translational toxicology in zebrafish
Curr. Opin. Toxicol. 23-24 , 56 - 66 10.1016/j.cotox.2020.05.004 - Teixidó, E., Leuthold, D., de Crozé, N., Léonard, M., Scholz, S. (2020):
Comparative assessment of the sensitivity of fish early‐life stage, Daphnia and algae to the chronic ecotoxicity of xenobiotics – perspectives for alternatives to animal testing
Environ. Toxicol. Chem. 39 (1), 30 - 41 10.1002/etc.4607 - Ulrich, N., Schweiger, N., Pfennigsdorff, A., Scholz, S., Goss, K.-U. (2020):
Yolk–water partitioning of neutral organic compounds in the model organism Danio rerio
Environ. Toxicol. Chem. 39 (8), 1506 - 1516 10.1002/etc.4744
2019 (8)
- Catron, T.R., Gaballah, S., Tal, T. (2019):
Using zebrafish to investigate interactions between xenobiotics and microbiota
Curr. Pharmacol. Rep. 5 , 468 - 480 10.1007/s40495-019-00203-7 - Halbach, K., Wagner, S., Scholz, S., Luckenbach, T., Reemtsma, T. (2019):
Elemental imaging (LA-ICP-MS) of zebrafish embryos to study the toxicokinetics of the acetylcholinesterase inhibitor naled
Anal. Bioanal. Chem. 411 (3), 617 - 627 10.1007/s00216-018-1471-2 - Küster, E., Kalkhof, S., Aulhorn, S., von Bergen, M., Gündel, U. (2019):
Effects of five substances with different modes of action on cathepsin H, C and L activities in zebrafish embryos
Int. J. Environ. Res. Public Health 16 (20), art. 3956 10.3390/ijerph16203956 - Leuthold, D., Klüver, N., Altenburger, R., Busch, W. (2019):
Can environmentally relevant neuroactive chemicals specifically be detected with the locomotor response test in zebrafish embryos?
Environ. Sci. Technol. 53 (1), 482 - 493 10.1021/acs.est.8b04327 - Ogungbemi, A., Leuthold, D., Scholz, S., Küster, E. (2019):
Hypo- or hyperactivity of zebrafish embryos provoked by neuroactive substances: a review on how experimental parameters impact the predictability of behavior changes
Environ. Sci. Eur. 31 , art. 88 10.1186/s12302-019-0270-5 - Perkins, E.J., Ashauer, R., Burgoon, L., Conolly, R., Landesmann, B., Mackay, C., Murphy, C.A., Pollesch, N., Wheeler, J.R., Zupanic, A., Scholz, S. (2019):
Building and applying quantitative adverse outcome pathway models for chemical hazard and risk assessment
Environ. Toxicol. Chem. 38 (9), 1850 - 1865 10.1002/etc.4505 - Philipp, K., Lemke, F., Scholz, S., Wallrabe, U., Wapler, M.C., Koukourakis, N., Czarske, J.W. (2019):
Diffraction-limited axial scanning in thick biological tissue with an aberration-correcting adaptive lens
Sci. Rep. 9 , art. 9532 10.1038/s41598-019-45993-4 - Teixidó, E., Kießling, T.R., Krupp, E., Quevedo, C., Muriana, A., Scholz, S. (2019):
Automated morphological feature assessment for zebrafish embryo developmental toxicity screens
Toxicol. Sci. 167 (2), 438 - 449 10.1093/toxsci/kfy250
2018 (9)
- Birke, A., Scholz, S. (2018):
Zebrafish embryo and acute fish toxicity test show similar sensitivity for narcotic compounds
ALTEX-Altern. Anim. Exp. 36 (1), 131 - 135 10.14573/altex.1808101 - Fischer, F.C., Abele, C., Droge, S.T.J., Henneberger, L., König, M., Schlichting, R., Scholz, S., Escher, B.I. (2018):
Cellular uptake kinetics of neutral and charged chemicals in in vitro assays measured by fluorescence microscopy
Chem. Res. Toxicol. 31 (8), 646 - 657 10.1021/acs.chemrestox.8b00019 - Jarque, S., Fetter, E., Veneman, W.J., Spaink, H.P., Peravali, R., Strähle, U., Scholz, S. (2018):
An automated screening method for detecting compounds with goitrogenic activity using transgenic zebrafish embryos
PLOS One 13 (8), e0203087 10.1371/journal.pone.0203087 - Larras, F., Billoir, E., Baillard, V., Siberchicot, A., Scholz, S., Wubet, T., Tarkka, M., Schmitt-Jansen, M., Delignette-Muller, M.-L. (2018):
DRomics: A turnkey tool to support the use of the dose–response framework for omics data in ecological risk assessment
Environ. Sci. Technol. 52 (24), 14461 - 14468 10.1021/acs.est.8b04752 - Legradi, J.B., Di Paolo, C., Kraak, M.H.S., van der Geest, H.G., Schymanski, E.L., Williams, A.J., Dingemans, M.M.L., Massei, R., Brack, W., Cousin, X., Begout, M.-L., van der Oost, R., Carion, A., Suarez‑Ulloa, V., Silvestre, F., Escher, B.I., Engwall, M., Nilén, G., Keiter, S.H., Pollet, D., Waldmann, P., Kienle, C., Werner, I., Haigis, A.-C., Knapen, D., Vergauwen, L., Spehr, M., Schulz, W., Busch, W., Leuthold, D., Scholz, S., vom Berg, C.M., Basu, N., Murphy, C.A., Lampert, A., Kuckelkorn, J., Grummt, T., Hollert, H. (2018):
An ecotoxicological view on neurotoxicity assessment
Environ. Sci. Eur. 30 , art. 46 10.1186/s12302-018-0173-x - Scholz, S., Schreiber, R., Armitage, J., Mayer, P., Escher, B.I., Lidzba, A., Léonard, M., Altenburger, R. (2018):
Meta‐analysis of fish early life stage tests—Association of toxic ratios and acute‐to‐chronic ratios with modes of action
Environ. Toxicol. Chem. 37 (4), 955 - 969 10.1002/etc.4090 - Sobanska, M., Scholz, S., Nyman, A.-M., Cesnaitis, R., Gutierrez Alonso, S., Klüver, N., Kühne, R., Tyle, H., de Knecht, J., Dang, Z., Lundbergh, I., Carlon, C., De Coen, W. (2018):
Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH)
Environ. Toxicol. Chem. 37 (3), 657 - 670 10.1002/etc.4055 - Teixido, E., Kerkhof, O., Kießling, T., Scholz, S. (2018):
Retrieving mode of action information by automated effect pattern analysis using the zebrafish embryo toxicity test
Reprod. Toxicol. 80 , 18 - 19 10.1016/j.reprotox.2018.06.074 - Teixidó, E., Krupp, E., Amberg, A., Czich, A., Scholz, S. (2018):
Species-specific developmental toxicity in rats and rabbits: Generation of a reference compound list for development of alternative testing approaches
Reprod. Toxicol. 76 , 93 - 102 10.1016/j.reprotox.2018.01.005
2017 (4)
- Andrade, T.S., Henriques, J.F., Almeida, A.R., Soares, A.M.V.M., Scholz, S., Dominigues, I. (2017):
Zebrafish embryo tolerance to environmental stress factors—Concentration–dose response analysis of oxygen limitation, pH, and UV-light irradiation
Environ. Toxicol. Chem. 36 (3), 682 - 690 10.1002/etc.3579 - Escher, B.I., Hackermüller, J., Polte, T., Scholz, S., Aigner, A., Altenburger, R., Böhme, A., Bopp, S.K., Brack, W., Busch, W., Chadeau-Hyam, M., Covaci, A., Eisenträger, A., Galligan, J.J., Garcia-Reyero, N., Hartung, T., Hein, M., Herberth, G., Jahnke, A., Kleinjans, J., Klüver, N., Krauss, M., Lamoree, M., Lehmann, I., Luckenbach, T., Miller, G.W., Müller, A., Phillips, D.H., Reemtsma, T., Rolle-Kampczyk, U., Schüürmann, G., Schwikowski, B., Tan, Y.-M., Trump, S., Walter-Rohde, S., Wambaugh, J.F. (2017):
From the exposome to mechanistic understanding of chemical-induced adverse effects
Environ. Int. 99 , 97 - 106 10.1016/j.envint.2016.11.029 - Kühnert, A., Vogs, C., Seiwert, B., Aulhorn, S., Altenburger, R., Hollert, H., Küster, E., Busch, W. (2017):
Biotransformation in the zebrafish embryo – temporal gene transcription changes of cytochrome P450 enzymes and internal exposure dynamics of the AhR binding xenobiotic benz[a]anthracene
Environ. Pollut. 230 , 1 - 11 10.1016/j.envpol.2017.04.083 - Teixido, E., Kießling, T., Leuthold, D., Scholz, S. (2017):
Effect signatures in zebrafish embryos exposed to compounds with potential developmental toxicity
Reprod. Toxicol. 72 , 49 10.1016/j.reprotox.2017.06.179
2016 (6)
- Klüver, N., Vogs, C., Altenburger, R., Escher, B.I., Scholz, S. (2016):
Development of a general baseline toxicity QSAR model for the fish embryo acute toxicity test
Chemosphere 164 , 164 - 173 10.1016/j.chemosphere.2016.08.079 - Scholz, S., Jarque, S., Fetter, E. (2016):
A fully automated screening method for detecting compounds with goitrogenic activity using transgenic zebrafish embryos
Reprod. Toxicol. 64 (Special Issue), 30 10.1016/j.reprotox.2016.06.064 - Scholz, S., Klüver, N., Kühne, R. (2016):
Analysis of the relevance and adequateness of using Fish Embryo Acute Toxicity (FET) Test Guidance (OECD 236) to fulfil the information requirements and addressing concerns under REACH. Report ECHA-UFZ contract ECHA/2014/341
Helmholtz Centre for Environmental Research - UFZ, Leipzig, 104 pp. - Shahid, M., Takamiya, M., Stegmaier, J., Middel, V., Gradl, M., Klüver, N., Mikut, R., Dickmeis, T., Scholz, S., Rastegar, S., Yang, L., Strähle, U. (2016):
Zebrafish biosensor for toxicant induced muscle hyperactivity
Sci. Rep. 6 , art. 23768 10.1038/srep23768 - Teixidó, E., Leuthold, D., Amberg, A., Czich, A., Krupp, E., Scholz, S. (2016):
ZFminus1: A strategy to reduce animal tests for developmental toxicity testing by a combined use of mammalian models and the zebrafish embryotoxicity test (ZFET or ZETA)
Reprod. Toxicol. 64 , 22 10.1016/j.reprotox.2016.06.048 - Teixidó, E., Leuthold, D., Scholz, S., Quevedo, C., Muriana, A., Czich, A., Krupp, E. (2016):
An inter-laboratory validation of the zebrafish embryo assay for the detection of developmental toxicity
Reprod. Toxicol. 64 , 44 10.1016/j.reprotox.2016.06.096
2015 (8)
- Fetter, E., Baldauf, L., Da Fonte, D.F., Ortmann, J., Scholz, S. (2015):
Comparative analysis of goitrogenic effects of phenylthiourea and methimazole in zebrafish embryos
Reprod. Toxicol. 57 , 10 - 20 10.1016/j.reprotox.2015.04.012 - Fetter, E., Smetanová, S., Baldauf, L., Lidzba, A., Altenburger, R., Schüttler, A., Scholz, S. (2015):
Identification and characterization of androgen-responsive genes in zebrafish embryos
Environ. Sci. Technol. 49 (19), 11789 - 11798 10.1021/acs.est.5b01034 - Hernández, R.B., Nishita, M.I., Espósito, B.P., Scholz, S., Michalke, B. (2015):
The role of chemical speciation, chemical fractionation and calcium disruption in manganese-induced developmental toxicity in zebrafish (Danio rerio) embryos
J. Trace Elem. Med. Biol. 32 , 209 - 217 10.1016/j.jtemb.2015.07.004 - Jonas, A., Scholz, S., Fetter, E., Sychrova, E., Novakova, K., Ortmann, J., Benisek, M., Adamovsky, O., Giesy, J.P., Hilscherova, K. (2015):
Endocrine, teratogenic and neurotoxic effects of cyanobacteria detected by cellular in vitro and zebrafish embryos assays
Chemosphere 120 , 321 - 327 10.1016/j.chemosphere.2014.07.074 - Klüver, N., König, M., Ortmann, J., Massei, R., Paschke, A., Kühne, R., Scholz, S. (2015):
Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds
Environ. Sci. Technol. 49 (11), 7002 - 7011 10.1021/acs.est.5b01910 - Luckenbach, T., Küster, E., Busch, W., Scholz, S., Altenburger, R. (2015):
What determines chemical uptake by the zebrafish embryo model?
Toxicol. Lett. 238 (2, Suppl.), S54 10.1016/j.toxlet.2015.08.151 - Massei, R., Vogs, C., Renner, P., Altenburger, R., Scholz, S. (2015):
Differential sensitivity in embryonic stages of the zebrafish (Danio rerio): The role of toxicokinetics for stage-specific susceptibility for azinphos-methyl lethal effects
Aquat. Toxicol. 166 , 36 - 41 10.1016/j.aquatox.2015.06.011 - Scholz, S. (2015):
In response: Quantitative adverse outcome pathways for prediction of adverse effects—An academic perspective
Environ. Toxicol. Chem. 34 (9), 1935 - 1937 10.1002/etc.3043
