Experimental Systems & Methods
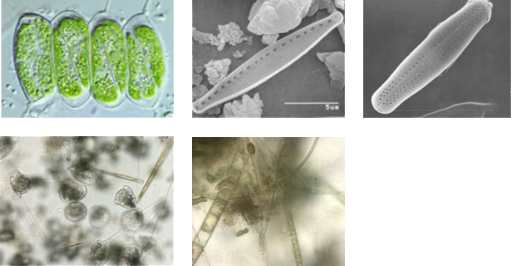
Analysis of acute and chronic effects in biofilms, green algae, cyanobacteria and diatoms
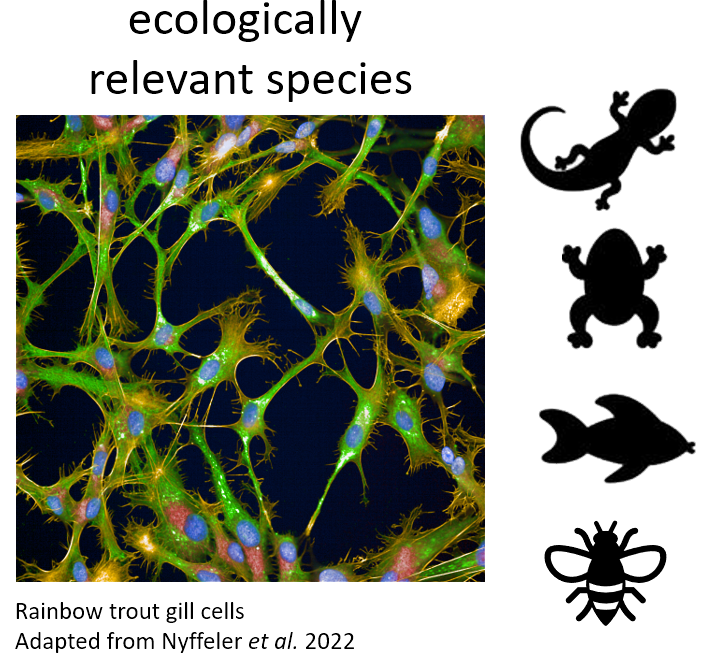
We use various celllines from different ecologically relevant species incl. human and fish for high throughput phenotypic profiling

We are using different invertebrate species e.g. daphnids (Daphnia magna), gammarids (Gammarus spec.), mosquitoes (Culex pipiens) and snails (Lymnaea stagnalis) for assessing short & long term effects as well as adaptation to mixtures (chemicals and/ or non-chemical stressors).
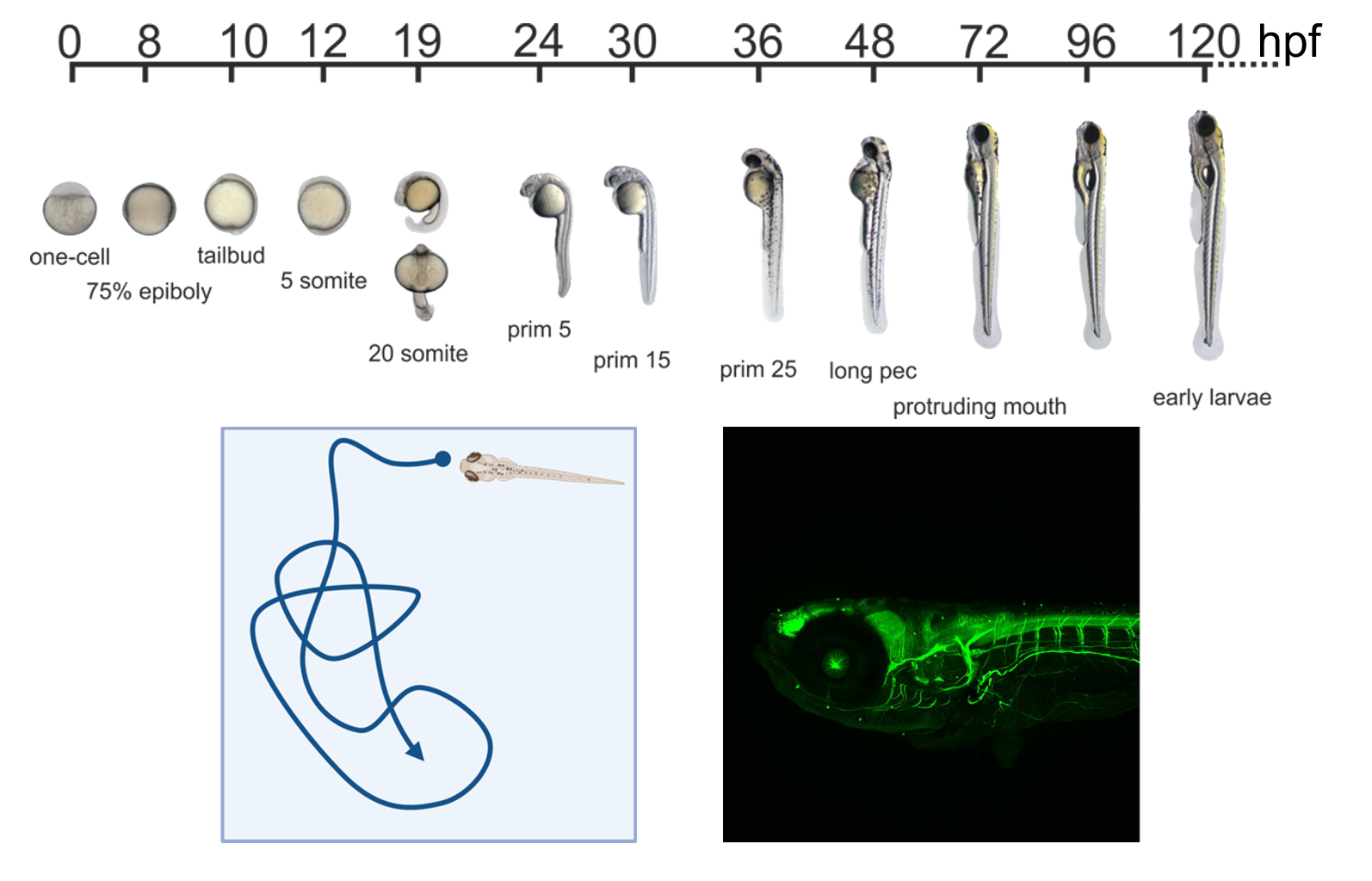
We use zebrafish embryos as a model organism due to their rapid external development, easy availability, and full transparency, enabling high-resolution phenotypic observation.

Measurement campaigns, recording of macroinvertebrates and pollution, water and sediment samples, continuous and event-related sampling.

We have 47 independent artificial streams, each 14 meters long and 0.4 meters wide, providing an ecologically relevant setup for complex experimental research. Water flows continuously, mimicking natural small streams. This ensures that organisms exhibit natural behaviours and ecological interactions.

For nano- and microcosm studies, we use different cultures of Daphnia magna and Culex pipiens or combinations of higher plants and biofilms to assess their responses to environmental stressors.

SPEAR Pesticide is optimized as a bioindicator for agricultural pesticides characterized by typical short-term pulse loads in flowing waters. The species characteristics considered include: (i) physiological sensitivity to insecticides and other pesticides with insecticidal effects, (ii) generation time, (iii) presence of aquatic life stages, and (iv) the ability to migrate and repopulate.

Understanding the influence of different landscape parameters on pesticide exposure in streams is important for risk assessment in order to improve exposure models and develop tailored mitigation strategies. By combining data from pesticide monitoring (e.g. from the German Kleingewässermonitoring – KgM) with information on the landscape, such as catchment characteristics, using GIS, we are working on validating existing exposure models and constructing new statistical models to explain and predict observed pesticide concentrations.

We use a FACS (Fluorescence-Activated Cell Sorting) system to efficiently count and sort algae in our samples. This system allows for precise quantification by sorting and detecting algae based on fluorescence characteristics, ensuring accurate analysis

We use HPLC with DAD (Diode-Array Detection) and FLD (Fluorescence Detection) for precise chemical analysis. This powerful combination allows for detailed separation, identification, and quantification of compounds, ensuring accurate and reliable results in our research.

We use an automated liquid handling platform which is designed for precise, efficient, and scalable sample processing in various laboratory applications.

We use an AI-powered automated imaging platform designed for rapid and high-content analysis of biological samples.
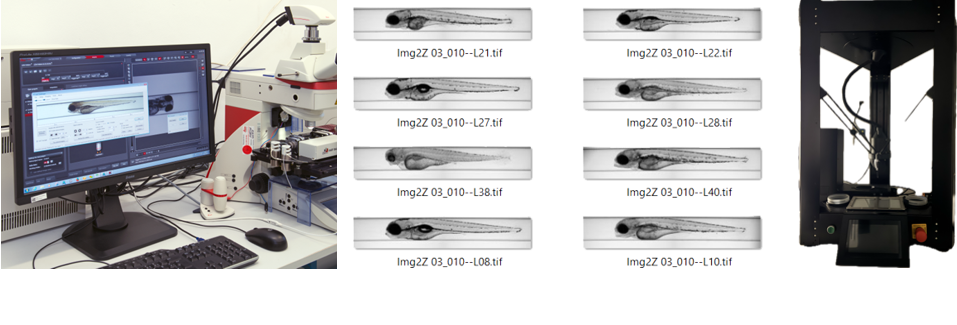
We are utilizing automated imaging systems (AIR and VAST) for zebrafish embryo effect profiling. This advanced setup enables high-throughput imaging and analysis, allowing for precise and efficient assessment of morphological effects incl. heart rate measurements.

We are utilizing ZebraBox systems from ViewPoint for automated zebrafish larval behavior tracking. This advanced platform enables high-throughput analysis of locomotion and stimulus responses, providing precise and reliable behavioral profiling for our research.

Our zebrafish facility uses the Tecniplast system to house various types of wildtype, transgenic and mutant fish. The system provides optimal housing conditions for this tropical species. With constant adjustment of the pH, temperature and salinity as well as regular checks of other important water parameters combined with regular health checks of our fish assure that our research using zebrafish embryos is reproducible and up to highest scientific standards
