
Research Focus:
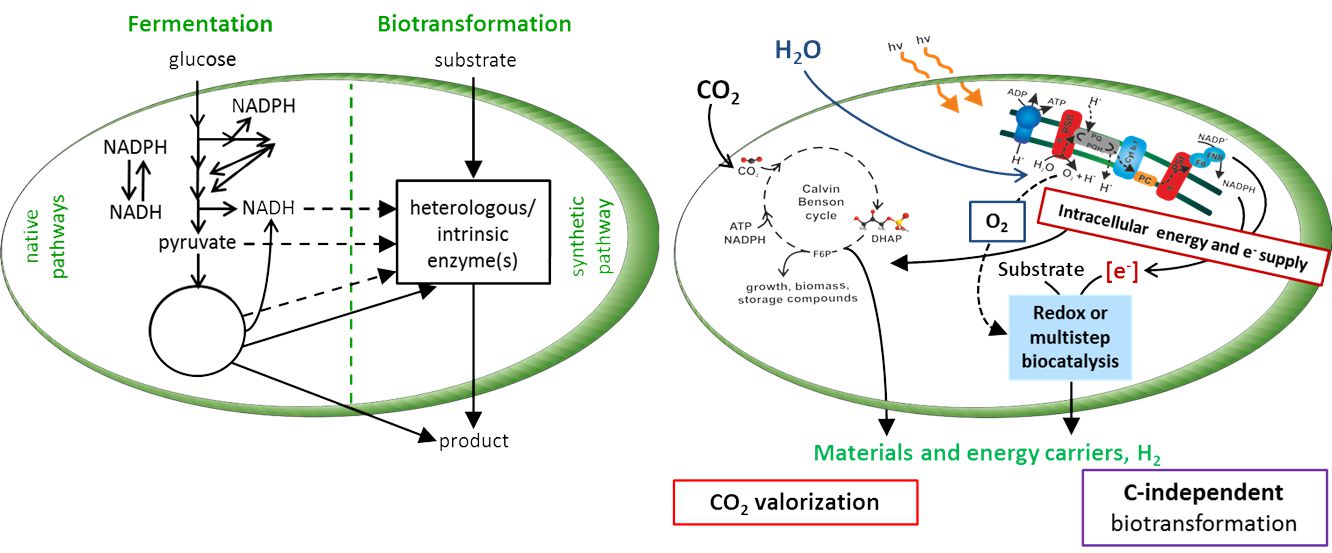
Research in the Applied Biocatalysis group focusses on whole-cell biocatalysis and aims at the development of efficient and stable bioprocesses via an integrated biocatalyst and reaction engineering approach. Specifically, redox biotransformations (oxygenases, dehydrogenases, hydrogenases), in vivo cascades / orthogonal pathways, and fermentative approaches are targeted via enzyme, metabolic, reaction, and process engineering to develop and apply engineered microbial cells for the eco-efficient production of bulk and fine chemicals as well as fuels and bioactives from renewable as well as fossil resources. The renewables considered include sunlight, water and CO2 and thus the exploitation of photosynthesis with H2 formation by means of O2-tolerant hydrogenases as a major focus. Cyanobacteria, E. coli, and Pseudomonads constitute the host strains mainly employed. Following a systems biotechnology approach, special emphasis lies on biocatalyst and process efficiency with the ultimate goal to develop environment-friendly and industrially feasible processes via rational biocatalyst and process engineering.
Group Leader:
Prof. Dr. Bruno Bühler
Academic Staff:
PhD-Students:
Bioprocess Engineer:
Guest Scientists:
Sara Lupacchini
Ana Camila Zenteno Illanes
Nina Antonia Siebert
Students / Hiwis:
Kashfia Jalil
Meera Dhaduk
Index:
You could use our publication index for further requests.
2025 (5)
- Bolay, P., Toepel, J., Bühler, B. (2025):
Biotechnological applications of cyanobacteria: Synechocystis and Synechococcus strains
In: Holtmann, D. (ed.)
Unconventional organisms in biotechnology
Adv. Biochem. Eng. Biotechnol. 192
Springer Nature, p. 155 - 191 10.1007/10_2025_282 - Janssen, K.N., Bolay, P., Tüllinghoff, A., Toepel, J., Spindler, D., Bühler, B., Lindberg, P. (2025):
Engineering cyanobacteria for high-yield photosynthetic isoprene production with long-term phenotypic stability
Plant Biotechnol. J. 10.1111/pbi.70395 - Lettau, E., Till, J., Toepel, J., Appel, J., Boehm, M., Sacco, D., Lorent, C., Teutloff, C., Mach, R.L., Gutekunst, K., Bühler, B., Lauterbach, L. (2025):
Engineering O2-tolerant chimeric hydrogenases optimized for ferredoxin coupling in Synechocystis sp. PCC 6803
ACS Synth. Biol. 14 (11), 4478 - 4495 10.1021/acssynbio.5c00494 - Lupacchini, S., Stauder, R., Opel, F., Klähn, S., Schmid, A., Bühler, B., Toepel, J. (2025):
Co-expression of auxiliary genes enhances the activity of a heterologous O2-tolerant hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803
Biotechnol. Biofuels Bioprod. 18 , art. 41 10.1186/s13068-025-02634-5 - Till, J., Toepel, J., Sacco, D., Bühler, B., Appel, J., Gutekunst, K. (2025):
Dataset for "Engineering O2-tolerant chimeric hydrogenases optimized for ferredoxin coupling in Synechocystis sp. PCC 6803" [Data set]
Zenodo 10.5281/zenodo.15839120
2024 (5)
- Bertelmann, C., Bühler, B. (2024):
Hin zu effizienter biokatalytischer Oxyfunktionalisierung von Steroiden [Towards efficient biocatalytic oxyfuntionalization of steroids]
Biospektrum 30 (5), 593 - 595 10.1007/s12268-024-2261-3 - Bertelmann, C., Bühler, B. (2024):
Strategies found not to be suitable for stabilizing high steroid hydroxylation activities of CYP450 BM3-based whole-cell biocatalysts
PLOS One 19 (9), e0309965 10.1371/journal.pone.0309965 - Bertelmann, C., Mock, M., Schmid, A., Bühler, B. (2024):
Efficiency aspects of regioselective testosterone hydroxylation with highly active CYP450-based whole-cell biocatalysts
Microb. Biotechnol. 17 (1), e14378 10.1111/1751-7915.14378 - Höhmann, S.M., Briol, T.A., Ihle, N., Frick, O., Schmid, A., Bühler, B. (2024):
Glycolate as alternative carbon source for Escherichia coli
J. Biotechnol. 381 , 76 - 85 10.1016/j.jbiotec.2024.01.001 - Tüllinghoff, A., Toepel, J., Bühler, B. (2024):
Enlighting electron routes in oxyfunctionalizing Synechocystis sp. PCC 6803
ChemBioChem 25 (6), e202300475 10.1002/cbic.202300475
2023 (3)
- Toepel, J., Karande, R., Bühler, B., Bühler, K., Schmid, A. (2023):
Photosynthesis driven continuous hydrogen production by diazotrophic cyanobacteria in high cell density capillary photobiofilm reactors
Bioresour. Technol. 373 , art. 128703 10.1016/j.biortech.2023.128703 - Toepel, J., Karande, R., Klähn, S., Bühler, B. (2023):
Cyanobacteria as whole-cell factories: current status and future prospectives
Curr. Opin. Biotechnol. 80 , art. 102892 10.1016/j.copbio.2023.102892 - Tüllinghoff, A., Djaya-Mbissam, H., Toepel, J., Bühler, B. (2023):
Light‐driven redox biocatalysis on gram‐scale in Synechocystis sp. PCC 6803 via an in vivo cascade
Plant Biotechnol. J. 21 (10), 2074 - 2083 10.1111/pbi.14113
2022 (6)
- Bertelmann, C., Mock, M., Koch, R., Schmid, A., Bühler, B. (2022):
Hydrophobic outer membrane pores boost testosterone hydroxylation by cytochrome P450 BM3 containing cells
Front. Catal. 2 , art. 887458 10.3389/fctls.2022.887458 - Bretschneider, L., Heuschkel, I., Bühler, K., Karande, R., Bühler, B. (2022):
Rational orthologous pathway and biochemical process engineering for adipic acid production using Pseudomonas taiwanensis VLB120
Metab. Eng. 70 , 206 - 217 10.1016/j.ymben.2022.01.014 - Grund, M., Jakob, T., Toepel, J., Schmid, A., Wilhelm, C., Bühler, B. (2022):
Heterologous lactate synthesis in Synechocystis sp. strain PCC 6803 causes a growth condition-dependent carbon sink effect
Appl. Environ. Microb. 88 (8), e00063-22 10.1128/aem.00063-22 - Opel, F., Siebert, N.A., Klatt, S., Tüllinghoff, A., Hantke, J.G., Toepel, J., Bühler, B., Nürnberg, D.J., Klähn, S. (2022):
Generation of synthetic shuttle vectors enabling modular genetic engineering of cyanobacteria
ACS Synth. Biol. 11 (5), 1758 - 1771 10.1021/acssynbio.1c00605 - Theodosiou, E., Tüllinghoff, A., Toepel, J., Bühler, B. (2022):
Exploitation of hetero- and phototrophic metabolic modules for redox-intensive whole-cell biocatalysis
Front. Bioeng. Biotechnol. 10 , art. 855715 10.3389/fbioe.2022.855715 - Tüllinghoff, A., Uhl, M.B., Nintzel, F.E.H., Schmid, A., Bühler, B., Toepel, J. (2022):
Maximizing photosynthesis-driven Baeyer-Villiger oxidation efficiency in recombinant Synechocystis sp. PCC6803
Front. Catal. 1 , art. 780474 10.3389/fctls.2021.780474
2021 (6)
- Bretschneider, L., Heuschkel, I., Ahmed, A., Bühler, K., Karande, R., Bühler, B. (2021):
Characterization of different biocatalyst formats for BVMO‐catalyzed cyclohexanone oxidation
Biotechnol. Bioeng. 118 (7), 2719 - 2733 10.1002/bit.27791 - Bretschneider, L., Heuschkel, I., Wegner, M., Lindmeyer, M., Bühler, K., Karande, R., Bühler, B. (2021):
Conversion of cyclohexane to 6-hydroxyhexanoic acid using recombinant Pseudomonas taiwanensis in a stirred-tank bioreactor
Front. Catal. 1 , art. 683248 10.3389/fctls.2021.683248 - Bretschneider, L., Wegner, M., Bühler, K., Bühler, B., Karande, R. (2021):
One-pot synthesis of 6-aminohexanoic acid from cyclohexane using mixed-species cultures
Microb. Biotechnol. 14 (3), 1011 - 1025 10.1111/1751-7915.13744 - Bühler, K., Bühler, B., Klähn, S., Krömer, J.O., Dusny, C., Schmid, A. (2021):
Biocatalytic production of white hydrogen from water using cyanobacteria
In: Rögner, M. (ed.)
Photosynthesis: Biotechnological applications with microalgae
De Gruyter, Berlin ; Boston, p. 279 - 306 10.1515/9783110716979-011 - Bühler, K., Krömer, J.O., Klähn, S., Bühler, B., Dusny, C., Schmid, A. (2021):
Weißer Wasserstoff made in Leipzig [White hydrogen made in Leipzig]
Biospektrum 27 (3), 335 10.1007/s12268-021-1572-x - Lupacchini, S., Appel, J., Stauder, R., Bolay, P., Klähn, S., Lettau, E., Adrian, L., Lauterbach, L., Bühler, B., Schmid, A., Toepel, J. (2021):
Rewiring cyanobacterial photosynthesis by the implementation of an oxygen-tolerant hydrogenase
Metab. Eng. 68 , 199 - 209 10.1016/j.ymben.2021.10.006
2020 (5)
- Schäfer, L., Bühler, K., Karande, R., Bühler, B. (2020):
Rational engineering of a multi-step biocatalytic cascade for the conversion of cyclohexane to polycaprolactone monomers in Pseudomonas taiwanensis
Biotechnol. J. 15 (11), art. 2000091 10.1002/biot.202000091 - Schäfer, L., Karande, R., Bühler, B. (2020):
Maximizing biocatalytic cyclohexane hydroxylation by modulating cytochrome P450 monooxygenase expression in P. taiwanensis VLB120
Front. Bioeng. Biotechnol. 8 , art. 140 10.3389/fbioe.2020.00140 - Till, P., Toepel, J., Bühler, B., Mach, R.L., Mach-Aigner, A.R. (2020):
Regulatory systems for gene expression control in cyanobacteria
Appl. Microbiol. Biotechnol. 104 (5), 1977 - 1991 10.1007/s00253-019-10344-w - Willrodt, C., Gröning, J.A.D., Nerke, P., Koch, R., Scholtissek, A., Heine, T., Schmid, A., Bühler, B., Tischler, D. (2020):
Highly efficient access to (S)-sulfoxides utilizing a promiscuous flavoprotein monooxygenase in a whole-cell biocatalyst format
ChemCatChem 12 (17), 4664 - 4671 10.1002/cctc.201901894 - Wohlgemuth, R., Bühler, B. (2020):
Molecular and engineering aspects of biocatalysis
Biotechnol. J. 15 (11), art. 2000499 10.1002/biot.202000499
2019 (8)
- Grund, M., Jakob, T., Wilhelm, C., Bühler, B., Schmid, A. (2019):
Electron balancing under different sink conditions reveals positive effects on photon efficiency and metabolic activity of Synechocystis sp. PCC 6803
Biotechnol. Biofuels 12 , art. 43 10.1186/s13068-019-1378-y - Heuschkel, I., Hoschek, A., Schmid, A., Bühler, B., Karande, R., Bühler, K. (2019):
Data on mixed trophies biofilm for continuous cyclohexane oxidation to cyclohexanol using Synechocystis sp. PCC 6803
Data Brief 25 , art. 104059 10.1016/j.dib.2019.104059 - Heuschkel, I., Hoschek, A., Schmid, A., Bühler, B., Karande, R., Bühler, K. (2019):
Mixed-trophies biofilm cultivation in capillary reactors
MethodsX 6 , 1822 - 1831 10.1016/j.mex.2019.07.021 - Hoschek, A., Bühler, B., Schmid, A. (2019):
Stabilization and scale‐up of photosynthesis‐driven ω‐hydroxylation of nonanoic acid methyl ester by two‐liquid phase whole‐cell biocatalysis
Biotechnol. Bioeng. 116 (8), 1887 - 1900 10.1002/bit.27006 - Hoschek, A., Heuschkel, I., Schmid, A., Bühler, B., Karande, R., Bühler, K. (2019):
Mixed-species biofilms for high-cell-density application of Synechocystis sp. PCC 6803 in capillary reactors for continuous cyclohexane oxidation to cyclohexanol
Bioresour. Technol. 282 , 171 - 178 10.1016/j.biortech.2019.02.093 - Hoschek, A., Toepel, J., Hochkeppel, A., Karande, R., Bühler, B., Schmid, A. (2019):
Light‐dependent and aeration‐independent gram‐scale hydroxylation of cyclohexane to cyclohexanol by CYP450 harboring Synechocystis sp. PCC 6803
Biotechnol. J. 14 (8), art. 1800724 10.1002/biot.201800724 - Ütkür, F.Ö., Schmid, A., Bühler, B. (2019):
Anaerobic C-H oxyfunctionalization: Coupling of nitrate reduction and quinoline hydroxylation in recombinant Pseudomonas putida
Biotechnol. J. 14 (8), art. 1800615 10.1002/biot.201800615 - Volmer, J., Lindmeyer, M., Seipp, J., Schmid, A., Bühler, B. (2019):
Constitutively solvent-tolerant Pseudomonas taiwanensis VLB120ΔCΔttgV supports particularly high styrene epoxidation activities when grown under glucose excess conditions
Biotechnol. Bioeng. 116 (5), 1089 - 1101 10.1002/bit.26924
2018 (1)
- Hoschek, A., Schmid, A., Bühler, B. (2018):
In situ O2 generation for biocatalytic oxyfunctionalization reactions
ChemCatChem 10 (23), 5366 - 5371 10.1002/cctc.201801262
2017 (6)
- Hoschek, A., Bühler, B., Schmid, A. (2017):
Overcoming the gas-liquid mass transfer of oxygen by coupling photosynthetic water oxidation with biocatalytic oxyfunctionalization
Angew. Chem.-Int. Edit. 56 (47), 15146 - 15149 10.1002/anie.201706886 - Kadisch, M., Julsing, M.K., Schrewe, M., Jehmlich, N., Scheer, B., von Bergen, M., Schmid, A., Bühler, B. (2017):
Maximization of cell viability rather than biocatalyst activity improves whole-cell ω-oxyfunctionalization performance
Biotechnol. Bioeng. 114 (4), 874 - 884 10.1002/bit.26213 - Kadisch, M., Schmid, A., Bühler, B. (2017):
Hydrolase BioH knockout in E. coli enables efficient fatty acid methyl ester bioprocessing
J. Ind. Microbiol. Biotechnol. 44 (3), 339 - 351 10.1007/s10295-016-1890-z - Kadisch, M., Willrodt, C., Hillen, M., Bühler, B., Schmid, A. (2017):
Maximizing the stability of metabolic engineering-derived whole-cell biocatalysts
Biotechnol. J. 12 (8), art. 1600170 10.1002/biot.201600170 - Theodosiou, E., Breisch, M., Julsing, M.K., Falcioni, F., Bühler, B., Schmid, A. (2017):
An artificial TCA cycle selects for efficient α-ketoglutarate dependent hydroxylase catalysis in engineered Escherichia coli
Biotechnol. Bioeng. 114 (7), 1511 - 1520 10.1002/bit.26281 - Volmer, J., Schmid, A., Bühler, B. (2017):
The application of constitutively solvent-tolerant P. taiwanensis VLB120ΔCΔttgV for stereospecific epoxidation of toxic styrene alleviates carrier solvent use
Biotechnol. J. 12 (7), art. 1600558 10.1002/biot.201600558
2016 (2)
- Ladkau, N., Assmann, M., Schrewe, M., Julsing, M.K., Schmid, A., Bühler, B. (2016):
Efficient production of the Nylon 12 monomer ω-aminododecanoic acid methyl ester from renewable dodecanoic acid methyl ester with engineered Escherichia coli
Metab. Eng. 36 , 1 - 9 10.1016/j.ymben.2016.02.011 - Willrodt, C., Hoschek, A., Bühler, B., Schmid, A., Julsing, M.K. (2016):
Decoupling production from growth by magnesium sulfate limitation boosts de novo limonene production
Biotechnol. Bioeng. 113 (6), 1305 - 1314 10.1002/bit.25883
2015 (5)
- Lindmeyer, M., Jahn, M., Vorpahl, C., Müller, S., Schmid, A., Bühler, B. (2015):
Variability in subpopulation formation propagates into biocatalytic variability of engineered Pseudomonas putida strains
Front. Microbiol. 6 , art. 1042 10.3389/fmicb.2015.01042 - Lindmeyer, M., Meyer, D., Kuhn, D., Bühler, B., Schmid, A. (2015):
Making variability less variable: matching expression system and host for oxygenase-based biotransformations
J. Ind. Microbiol. Biotechnol. 42 (6), 851 - 866 10.1007/s10295-015-1615-8 - Theodosiou, E., Frick, O., Bühler, B., Schmid, A. (2015):
Metabolic network capacity of Escherichia coli for Krebs cycle-dependent proline hydroxylation
Microb. Cell. Fact. 14 , art. 108 10.1186/s12934-015-0298-1 - Volmer, J., Schmid, A., Bühler, B. (2015):
Guiding bioprocess design by microbial ecology
Curr. Opin. Microbiol. 25 , 25 - 32 10.1016/j.mib.2015.02.002 - Willrodt, C., Hoschek, A., Bühler, B., Schmid, A., Julsing, M.K. (2015):
Coupling limonene formation and oxyfunctionalization by mixed-culture resting cell fermentation
Biotechnol. Bioeng. 112 (9), 1738 - 1750 10.1002/bit.25592

Project: AI-supported biotechnology for resource-efficient active compound and bio-nylon production (BIOWIN)
(JTF InfraProNet 2021-2027, proposal-no. 100702818)
The project aims to develop innovative and sustainable technologies for the production of the antifibrinolytic agent ε-aminocaproic acid and bio-nylon monomers. The production strategies are based on the same artificial metabolic pathways, which will be optimized to maximize production rates. E. coli, Pseudomonads, and cyanobacterial strains are used or developed as production strains. Reaction technology and process concepts are being developed for these strains, including biofilm-based concepts. In order to accelerate process development, a paradigm shift from empirical to systematic approaches using AI and in-silico modeling will be implemented.

Project: New Green Chemistry a contribution to a sustainable bioeconomy
(Proposal-no: 100328904)
The action New Green Chemistry extends the intrastructure at the Helmholtz Centre for Environmental Research GmbH – UFZ.
The "New Green Chemistry" approach aims at new processes, which consistently move ressources such as minerals and CO2 in circles, do not utilize agricultural areas and only cause minor climate-relevant emissions. Thereby, CO2 is assimilated by algae, but not to form biomass, but the organic acid glycolate. This acid is then used in a second process step in place of glucose and without harvesting and purification efforts for the microbial production of building blocks of the chemical industry. Prospectively, largely all products, which today are based on glucose (or starch) as carbon source, may be produced in this way.
The bioreactor equipment financed by this project will be utilized for the cultivation of heterotrophic microorganisms to develop the second process step and evaluate the coupling of both process steps.
Lecture: “Basics of Biocatalysis” in the 1st semester module “Introduction to Chemical Biotechnology” and "Drug target identification and validation"
Lecturer: Prof. Bruno Bühler
When: Fridays in the winter semester, 1 pm – 3 pm or according to announcement
Where: MLU Halle, Weinberg-Campus, Hoher Weg 8, SR 101 HW or SR 107 HW according to announcement
---------------------
Lecture: “Whole-Cell Biocatalysis” in the 3rd semester module “Applied Biocatalysis”
Lecturer: Prof. Bruno Bühler
When: Fridays in the winter semester, 10 am - 12 am or according to announcement
Where: MLU Halle, Weinberg-Campus, Hoher Weg 8, SR 101 HW or
SR 107 HW according to announcement
---------------------
Project Seminar/ practical course on Applied Biocatalysis
Responsible Supervisor: Prof. Bruno Bühler
When: One week in the winter semester break according to announcement
Where: UFZ, Leipzig
Master thesis 1:
Light-driven terminal hydroxylation of medium-chain alkanes using recombinant strains of Synechocystis sp.
supervisors: Dr. Adrian Tüllinghoff and Prof. Dr. Bruno Bühler
contact: adrian.tuellinghoff@ufz.de, bruno.buehler@ufz.de
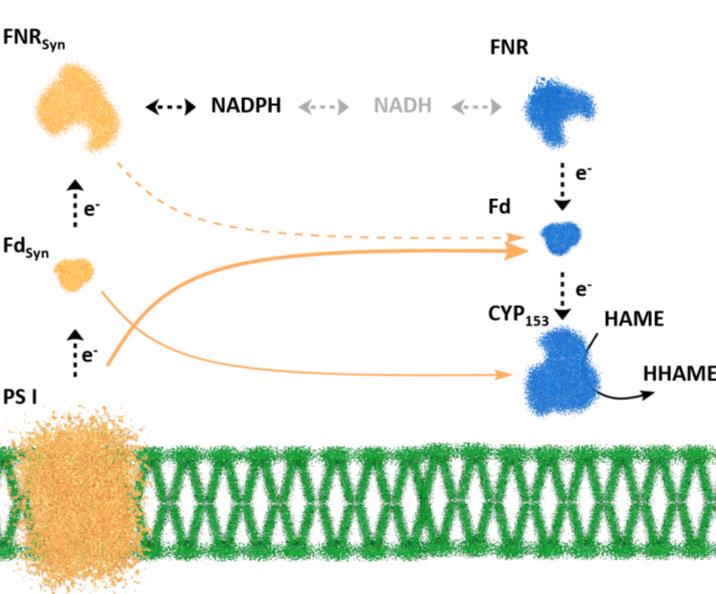
Background: Terminal hydroxylation of medium-chain alkanes is a highly-desired enzymatic step for the production of polymer precursors from renewable fatty-acid methylesters (FAMEs).1 This oxygenation reaction can be conducted in a sustainable fashion when employing photosynthetically active microorganisms, such as cyanobacteria, as whole-cell biocatalysts. Coupling heterologous redox reactions to the photosynthetic light reaction in recombinant cyanobacteria gives access to light as energy and water as electron (and also O2) source.2 Previous studies showed that electron transfer from the photosynthetic apparatus to heterologous consumers, such as cytochrome P450 monooxygenases (CYP), is supported by host-intrinsic electron carriers providing electrons to the heterologous enzymes at high rates.3
Scope: Strains of the cyanobacterium Synechocystis sp. PCC 6803 expressing specific CYPs of the subfamily CYP153 are to be screened for terminal hydroxylation of hexanoic acid methyl ester (HAME). Natively, these CYPs are three-component systems, i.e., they consist of a NADH:ferredoxin-reductase (FNR), a ferredoxin (Fd), and the active oxygenase subunit. In Synechocystis sp. PCC 6803, a versatile set of host-intrinsic electron carriers was shown to (at least partly) take over the role of the native electron transfer partners FNR and Fd of a specific CYP from Acidovorax. By omitting FNR and/or Fd, it will be tested, if this compensation also occurs for the CYP153 enzymes used here. Next, new combinations of suitable Fd, FNR, and CYPs will be tested in vivo to optimize electron transfer towards product formation. The strain with optimized electron route will be tested in photobioreactors to examine feasibility at lab-scale.
Techniques:
- Biochemical techniques (activity assays, expression studies, CO spectra)
- Analytical techniques (HPLC, GC-FID)
- Microbiology techniques (Cultivation of phototrophic cells, transformation, microscopy)
- Bioprocess development (Feeding strategies, bioreactor cultivation and control)
References:
1. Ladkau, N., Assmann, M., Schrewe, M., Julsing, M. K., Schmid, A., and Bühler, B. (2016) Efficient production of the Nylon 12 monomer omega-aminododecanoic acid methyl ester from renewable dodecanoic acid methyl ester with engineered Escherichia coli, Metab Eng 36, 1-9.
2. Tüllinghoff, A., Djaya-Mbissam, H., Toepel, J., and Bühler, B. (2023) Light-driven redox biocatalysis on gram-scale in Synechocystis sp. PCC 6803 via an in vivo cascade, Plant Biotechnol J 21, 2074-2083.
3. Tüllinghoff, A., Toepel, J., and Bühler, B. (2024) Enlighting Electron Routes In Oxyfunctionalizing Synechocystis sp. PCC 6803, ChemBioChem 25, e202300475.
Master thesis 2:
Enhancing light-driven oxygenase biocatalysis by using membrane uncouplers for metabolic balancing
supervisors: Dr. Adrian Tüllinghoff and Prof. Dr. Bruno Bühler
contact: adrian.tuellinghoff@ufz.de, bruno.buehler@ufz.de
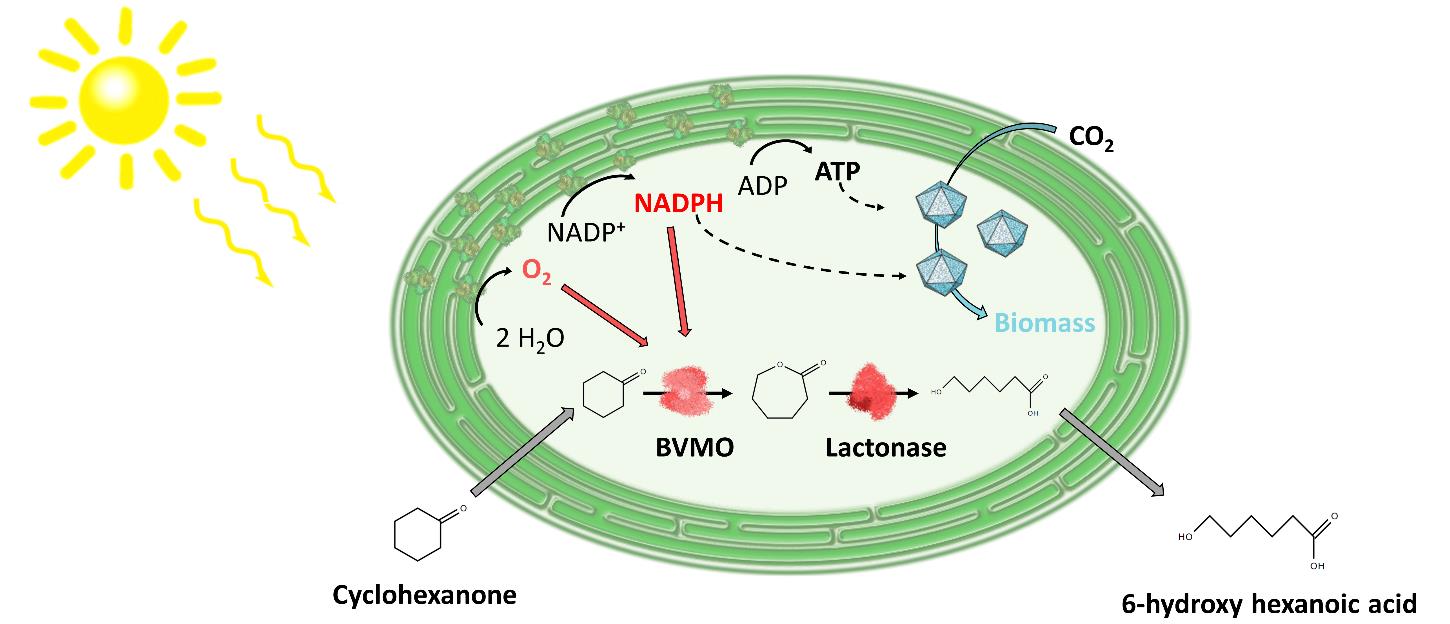
Background: To produce chemicals and goods in a truly sustainable and land-saving fashion, photo-biotechnology is a highly promising option.1 The photosynthetic apparatus of phototrophs enables the conversion of light into usable cellular energy. Cyanobacteria are ideal hosts for light-driven biocatalysis. Electron consuming biotransformations, such as oxygenase reactions, can be closely linked to the photosynthetic light reaction, giving access to light as energy and water as electron source.2, 3 The one-sided withdrawal of electron equivalents, such as NADPH, for heterologous redox reactions, however, is prone to affect photosynthetic metabolism.4 To reconstitute a more balanced ratio of ATP and NADPH levels, membrane uncouplers (uncoupling the proton gradient over the thylakoid membrane to reduce ATP formation) may help.
Scope: The impact of specific membrane uncouplers on the growth and the physiology of the model cyanobacterium Synechocystis sp. PCC 6803 is to be tested in different cultivation setups such as shaking flasks, bubble columns, and stirred-tank reactors. Further, options to enhance the biocatalytic performance of recombinant Synechocystis strains will be investigated, testing different uncoupler concentrations, time points of uncoupler supply and oxygenation rates. The findings at small scale will be transferred to photobioreactors at lab-scale to test the performance in an optimized production process. Depending on the progress, the project can be extended either by physiological characterization of the oxygenase-active biocatalyst or by testing downstream processing of the photo-biotechnological product.
Techniques:
- Biochemical techniques (activity assays, expression studies)
- Analytical techniques (HPLC, GC-FID, PAM fluorometry)
- Microbiology techniques (Cultivation of phototrophic cells, toxicity studies, microscopy)
- Bioprocess development (Feeding strategies, bioreactor cultivation and control)
References:
1. Brandenburg, F., Theodosiou, E., Bertelmann, C., Grund, M., Klähn, S., Schmid, A., and Krömer, J. O. (2021) Trans-4-hydroxy-L-proline production by the cyanobacterium Synechocystis sp. PCC 6803, Metab Eng Commun 12, e00155.
2. Hoschek, A., Toepel, J., Hochkeppel, A., Karande, R., Bühler, B., and Schmid, A. (2019) Light-dependent and aeration-independent gram-scale hydroxylation of cyclohexane to cyclohexanol by CYP450 harboring Synechocystis sp. PCC 6803, Biotechnol J 14, e1800724.
3. Tüllinghoff, A., Uhl, M. B., Nintzel, F. E., Schmid, A., Bühler, B., and Toepel, J. (2022) Maximizing photosynthesis-driven Baeyer-Villiger oxidation efficiency in recombinant Synechocystis sp. PCC 6803, Front Catal 1, art. 780474.
4. Tüllinghoff, A., Djaya-Mbissam, H., Toepel, J., and Bühler, B. (2023) Light-driven redox biocatalysis on gram-scale in Synechocystis sp. PCC 6803 via an in vivo cascade, Plant Biotechnol J 21, 2074-2083.
