Animal-free hazard assessment
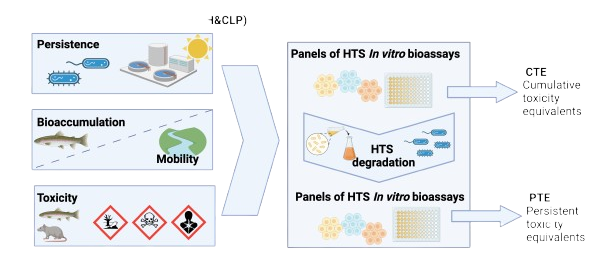
The European chemicals regulation REACH requires that substances of very high concern (SVHC) are identified based on hazard indicators, including persistence (P), bioaccumulation (B) and toxicity (T), as well as specific health hazards (i.e., carcinogenic, mutagenic and toxic for reproduction (CMR) and endocrine disruption (ED)). In 2023, the EU has introduced the latest revision of the regulation for Classification, Labelling and Packaging of substances and mixtures (CLP), which now also includes PBT and PMT with mobility (M) and ED as new hazard indicators. Testing these hazard properties is time-consuming, expensive and animal-intensive. Therefore, we work on developing modern animal-free hazard assessment methods that rely on batteries of in vitro bioassays for human health and the environment that are combined with high-throughput biodegradation assays to derive the modern hazard indicators Cumulative Toxicity Equivalents (CTE) and Persistent Toxicity Equivalents (PTE) that have the potential to replace existing PBT and consolidate with emerging PMT indicators.
(1) are the chemical or the mixture of chemicals toxic?
(2) is this toxicity persistent or will it be attenuated (or even aggravated) by environmental (bio)degradation?
More information: Escher, B. I.; Altenburger, R.; Blüher, M.; Colbourne, J. K.; Ebinghaus, R.; Fantke, P.; Hein, M.; Köck, W.; Kümmerer, K.; Leipold, S.; Li, X.; Scheringer, M.; Scholz, S.; Schloter, M.; Schweizer, P.-J.; Tal, T.; Tetko, I.; Traidl-Hoffmann, C.; Wick, L. Y.; Fenner, K., Modernizing persistence–bioaccumulation–toxicity (PBT) assessment with high throughput animal-free methods. Arch. Toxicol. 2023
