Biotechnology of metalloproteins and
BMBF Junior Research Group "CoMet"
Research group led by Dr. Darja Deobald

To gain deeper insights into the organization and functionality of this protein complex, we employ a range of advanced biochemical techniques. These include detergent-based solubilization, purification and enrichment using various chromatographic methods, blue native-PAGE, and heterologous production. These efforts are further complemented by mass spectrometry to analyze and characterize the complex in detail.
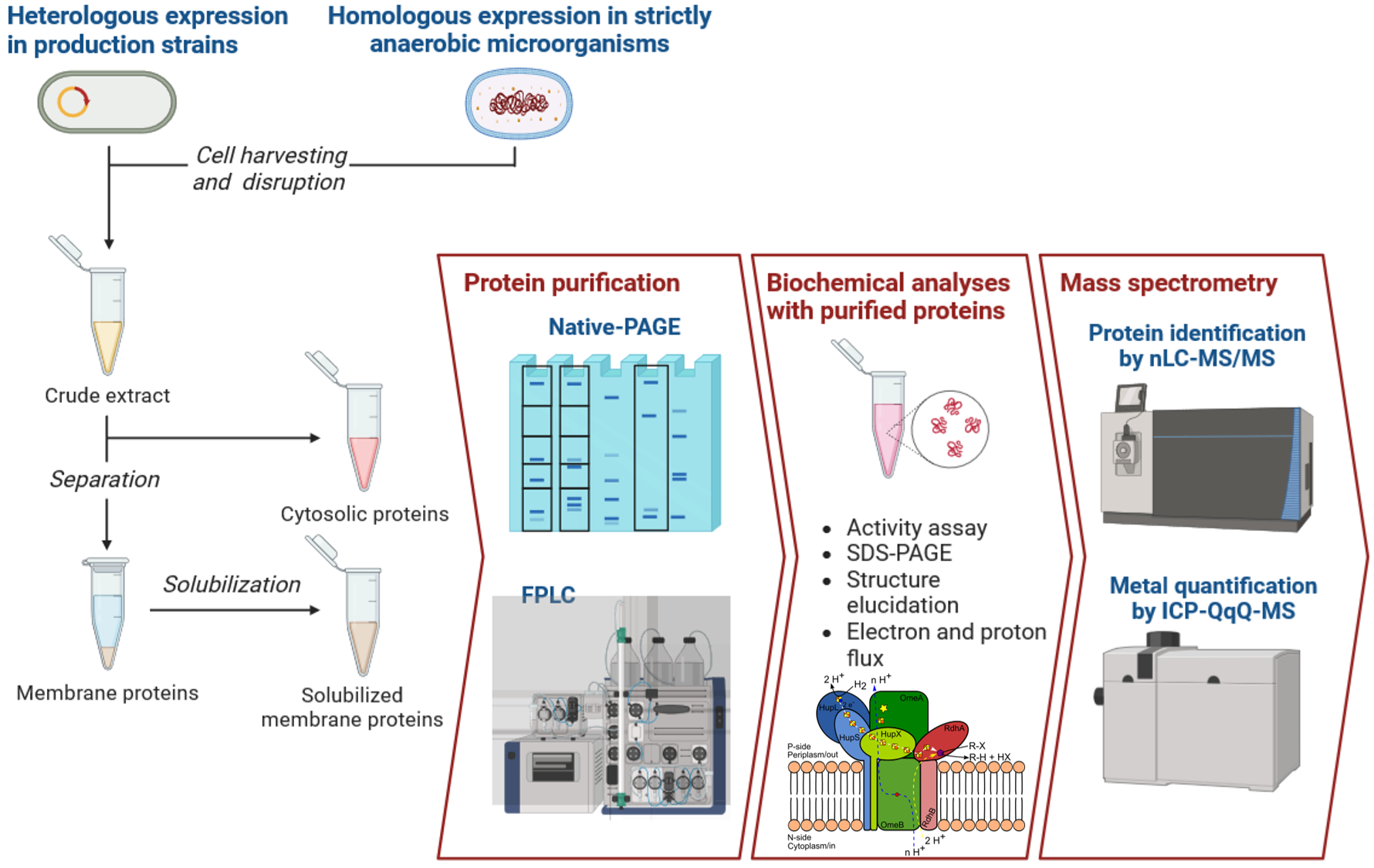
We are also deeply interested in studying metabolic processes that are
highly conserved in microorganisms with ancient traits, such as Dehalococcoidia.
By identifying, purifying, and characterizing the proteins involved in
these "primordial" metabolic pathways, we aim to illuminate the early
evolution of proteins and life itself. This research allows us to better
understand how anoxic processes, along with specific proteins,
contributed to the origin and evolution of life and how they continue to
influence natural and artificial matter cycles today.
Ultimately,
our group seeks to develop foundational concepts across various
scientific fields based on insights gained from our fundamental
research. We strive to translate these concepts into practical
applications, bridging the gap between basic science and real-world
systems.
The "CoMet" project, funded by BMBF (grant number: 031B1507) aims to explore a modular and adaptable cobalamin (B12)-dependent methylation system in which the B12 shuttle protein serves as a central mediator, transferring methyl groups between various donor and acceptor molecules. This system should enable diverse biological methylation reactions in a highly modular manner.

The proposed B12 methylation system offers a sustainable approach to biological methylation processes by utilizing simple C1 compounds, such as methanol or acetate, as methyl donors for methyltransferase I. These C1 compounds can be produced on an industrial scale via electrochemical CO2 reduction, effectively functioning as a CO2 sink. Methyltransferase I facilitates the methylation of the B12 shuttle protein, which in turn drives subsequent methylation reactions.
Additionally, this system reduces dependency on fossil raw materials by incorporating substrates derived from microbial processes, such as medium- and long-chain fatty acids and alcohols, as well as renewable resources and waste streams. These substrates can serve as methyl acceptors for methyltransferase II, which is responsible for transferring methyl groups to a variety of acceptor molecules, further broadening the system’s applicability and environmental benefits.
Our laboratory is equipped with a high-resolution mass spectrometer (nLC-MS/MS, Thermo Orbitrap Fusion) for the identification of proteins and metabolites, along with advanced data processing pipelines. We are pleased to offer measurement time for collaborations on environmental topics, whether through scientific partnerships or on a commercial basis.
Group members
Students are warmly welcome to take part in our research during their Master or Bachelor thesis projects. We accept unsolicited applications at any time at darja.deobald@ufz.de
Scientists
PhD students
Technician
Master and Bachelor students
Sarina Esfandiari
Ella Heyer
Jolina Hertz
Former students and members
Mirrah Matni Binti Mohd Sharif
Raphael Kaiser
Max Alexander Klamke
Rebecca Klemencic
Julian Waldhauer
Muhammad Jan Brohi
