CITEPro
Chemicals In The Environment Profiler
5 Exposure assessment in bioassays
The CITEPro module “Exposure assessment in bioassays” aims at the direct combination of the bioassay testing in module 1 with instrumental analysis. The effects measured in in vitro bioassays are typically reported as nominal effect concentrations. However, various processes, e.g. sorption to plastic well plates, binding to medium components, metabolism, and volatilization can influence the bioavailability of the test chemicals in in vitro bioassays. The free concentration (Cfree) in the assay medium is regarded a better dose metric than the nominal concentration. In serum-free media Cfree can be determined directly, while a solid phase microextraction (SPME) method is used for the cell-based bioassays of module 1 to remove the sample matrix. All samples are analyzed using an LC-MS/MS system from Agilent. The experimental exposure assessment is accompanied by physicochemical characterization of the test chemicals. A Sirius T3 from Pion Inc. will be used for the determina-tion of acidity constants, pH dependent solubility and octanol-water distribution ratios of ionizable test chemicals.
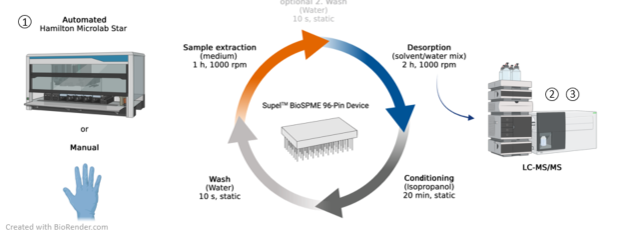
Application: Free concentration measurement in cell-based bioassays for quantitative in vitro to in vivo extrapolation and determination of partition constants
Main components
- CITEPro high-throughput bioassay infrastructure
- LC-MS/MS from Agilent with Online-SPE option and ESI and APCI source
- HPLC-UV/FLD
- Various Highspeed shakers from QInstrument
Selected literature references
Henneberger, L., J. Huchthausen, N. Wojtysiak and B. I. Escher (2021). "Quantitative In Vitro-to-In Vivo Extrapolation: Nominal versus Freely Dissolved Concentration." Chemical Research in Toxicology 34(4): 1175-1182.
Henneberger, L., M. Mühlenbrink, M. König, R. Schlichting, F. C. Fischer and B. I. Escher (2019). "Quantification of freely dissolved effect concentrations in in vitro cell-based bioassays." Arch Toxicol 93(8): 2295-2305.
Huchthausen, J., M. König, B. I. Escher and L. Henneberger (2023). "Experimental exposure assessment for in vitro cell-based bioassays in 96- and 384-well plates." Frontiers in Toxicology 5: 1221625.Huchthausen, J., M. Mühlenbrink, M. König, B. I. Escher and L. Henneberger (2020). "Experimental Exposure Assessment of Ionizable Organic Chemicals in In Vitro Cell-Based Bioassays." Chem Res Toxicol 33(7): 1845-1854.
Qin, W., L. Henneberger, J. Huchthausen, M. König and B. I. Escher (2023). "Role of bioavailability and protein binding of four anionic perfluoroalkyl substances in cell-based bioassays for quantitative in vitro to in vivo extrapolations." Environment International 173: 107857.

