CITEPro
Chemicals In The Environment Profiler
3 High-Throughput Chemoassays: Physicochemical
Properties, Reactivity, Abiotic Degradation & Biotransformation
The physicochemical properties of chemicals, such as solubility, speciation and hydrophobicity influence their distribution in biological systems. The Sirius T3 titrator measures these parameters in a high-throughput format for the most accurate chemical characterization. Chemoassays are used to the reactivity of single chemicals or complex mixtures. Electrophilic chemicals are able to react with nucleophilic target sites of biomolecules such as amino acids, peptides, proteins, or the genetic material, thus triggering a variety of adverse health effects. Chemoassays employ the tripeptide glutathione or amino acids as model nucleophiles to characterize the electrophilic reactivity of these chemicals in a HPLC-based high-throughput approach in terms of rate constants, percentage depletion rates or formed adduct patterns. Besides the abiotic reactivity of a chemical, biotransformation can lead to the formation of reactive metabolites with high relevance. Biotransformation is commonly assessed with animal-derived products (e.g., liver S9 or microsomes), but can also be achieved with a cost-efficient, fast and animal-free approach.
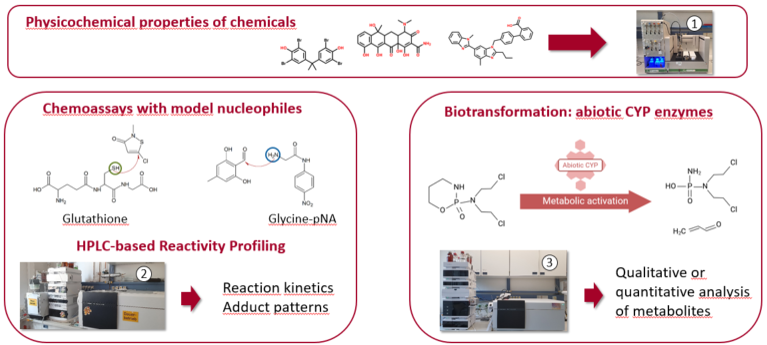
Application: Reactivity Assessment of Single Chemicals & Complex Mixtures
Main components
- Sirius T3 automatic platform for the determination of solubility, acidity constants and octanol-water partition constants
- HPLC-UV-Vis: reactivity of single chemicals & mixtures toward model nucleophiles mimicking reactive target sites in proteins. HPLC-UV-FLD: analysis of chemical metabolites
- HPLC-MS/MS to analyze adduct patterns to unravel drivers & mechanisms of reactive toxicity and for qualitative and quantitative analysis of chemical metabolites
Selected literature references
Böhme, A., Moldrickx, J., Schüürmann, G. (2021): "Amino reactivity of glutardialdehyde and monoaldehydes - chemoassay profile vs skin sensitization potency." Chem. Res. Toxicol. 34 (11), 2353 – 2365. 10.1021/acs.chemrestox.1c00266
Böhme, A., Ulrich, N., Schüürmann, G. (2023): "Amino chemoassay profiling of aromatic aldehydes–unraveling drivers of their skin sensitization potency." Chem. Res. Toxicol. 36 (7), 1055 – 1070. 10.1021/acs.chemrestox.3c00013
Huchthausen, J.; Escher, B. I.; Grasse, N.; Konig, M.; Beil, S.; Henneberger, L. (2023): "Reactivity of Acrylamides Causes Cytotoxicity and Activates Oxidative Stress Response." Chem. Res. Toxicol. 36 (8), 1374-1385. 10.1021/acs.chemrestox.3c00115
Henneberger, L.; Huchthausen, J.; Braasch, J.; König, M.; Escher, B. I. (2024): "In Vitro Metabolism and p53 Activation of Genotoxic Chemicals: Abiotic CYP Enzyme vs Liver Microsomes." Chem. Res. Toxicol. 37 (8), 1364-1373. DOI: 10.1021/acs.chemrestox.4c00101.

