Core expertise: Passive sampling
In many environmental applications, passive sampling (PS) is advantageous over exhaustive extraction techniques which are well established but are usually limited to providing a snapshot of the total concentration of chemicals present when collecting the sample. PS devices (PSDs) are versatile, robust, straightforward and inexpensive. A PSD is brought into contact with the environmental medium of study to allow the chemicals to passively diffuse to the sampler where they are enriched. Depending on the configuration, the chemicals in the PSD are either representative of the freely dissolved concentrations (Cfree) of chemicals, relevant for partitioning and uptake into biota, or give time-weighted average concentrations.
Different passive samplers have been developed for monitoring both legacy and emerging environmental pollutants from a wide range of chemical classes. Many PS targeting hydrophobic, bioaccumulative pollutants (3 < log Kow < 8) comprise a polymeric sampling phase, e.g. silicone or low-density polyethylene, that captures a broad range of non-polar organic pollutants. Other PS formats such as the Chemcatcher® (deployable with various sampling phases) focus on more polar organic compounds (log Kow < 4), ionic species and metal ions.
Both types of PS can either be employed in the kinetic uptake phase or be operated in equilibrium sampling mode (Figure 1). The media they can be applied for are numerous, including water, sediment/soil/suspended matter and biota. When operating in equilibrium mode, we refer to the PS as “chemometers”, which represent a common, universal and well-defined polymer reference phase for sampling of nonpolar organic pollutants across different matrices. Using such chemometers, we investigate amongst others abiotic media like sediment, aquatic organisms along trophic webs (e.g. mussels, non-predatory and predatory fish) and different tissues of marine mammals.
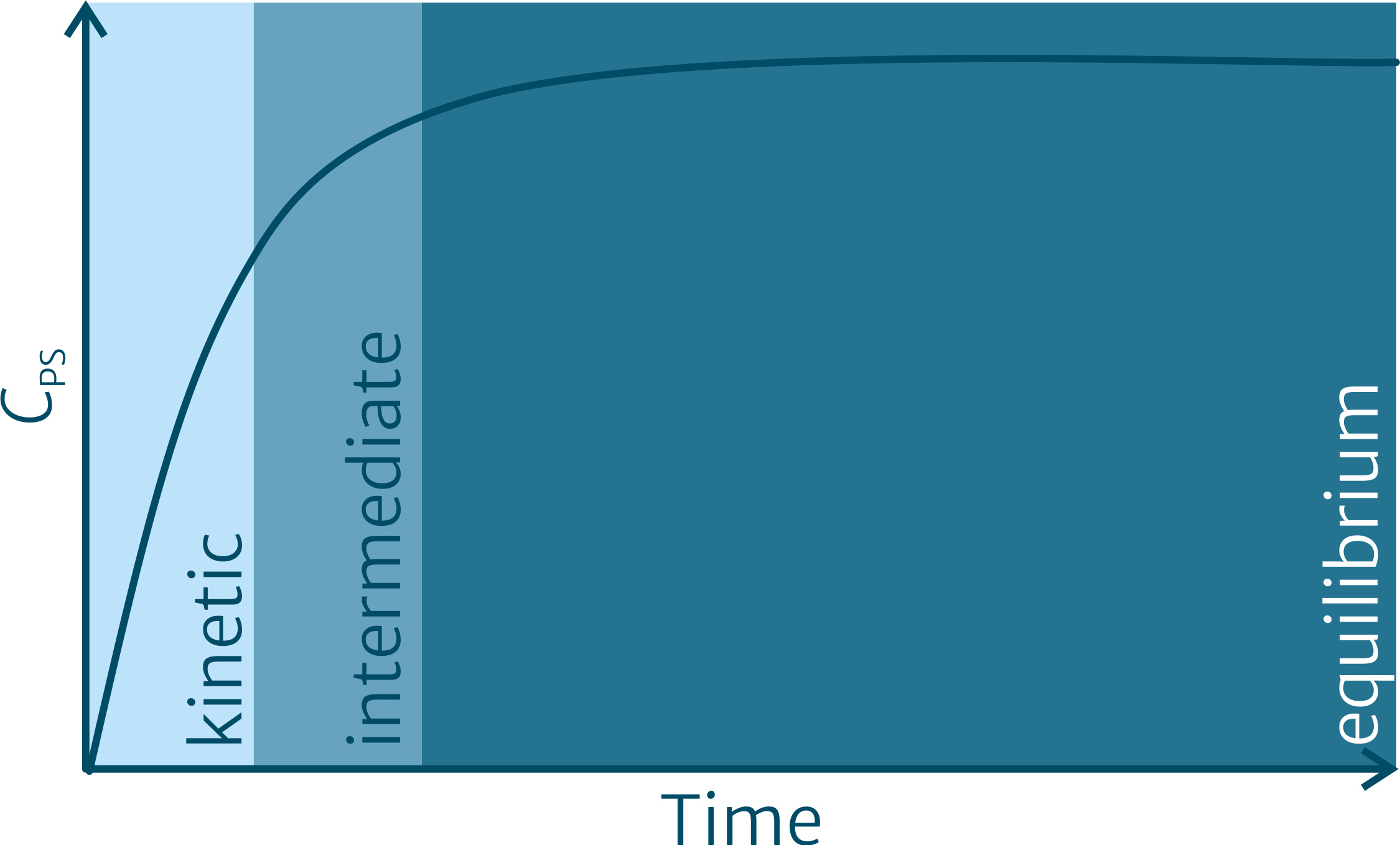
Figure 1: Typical uptake curve for the enrichment of a compound in the PS. Samplers are usually applied in the kinetic or in the equilibrium mode. The concentration in the sampled medium can be determined by applying the respective equations shown below.
Once the sampling is terminated, the PSD is cleaned, solvent-extracted and the extract is submitted to instrumental analysis or dosed into bioassays. The mass of chemicals accumulated in the PS, determined by instrumental analysis, is then used to calculate the concentrations in the medium of study as described, e.g., in the Feature Articles by Mayer et al. (2003) and Rojo-Nieto & Jahnke (2023).
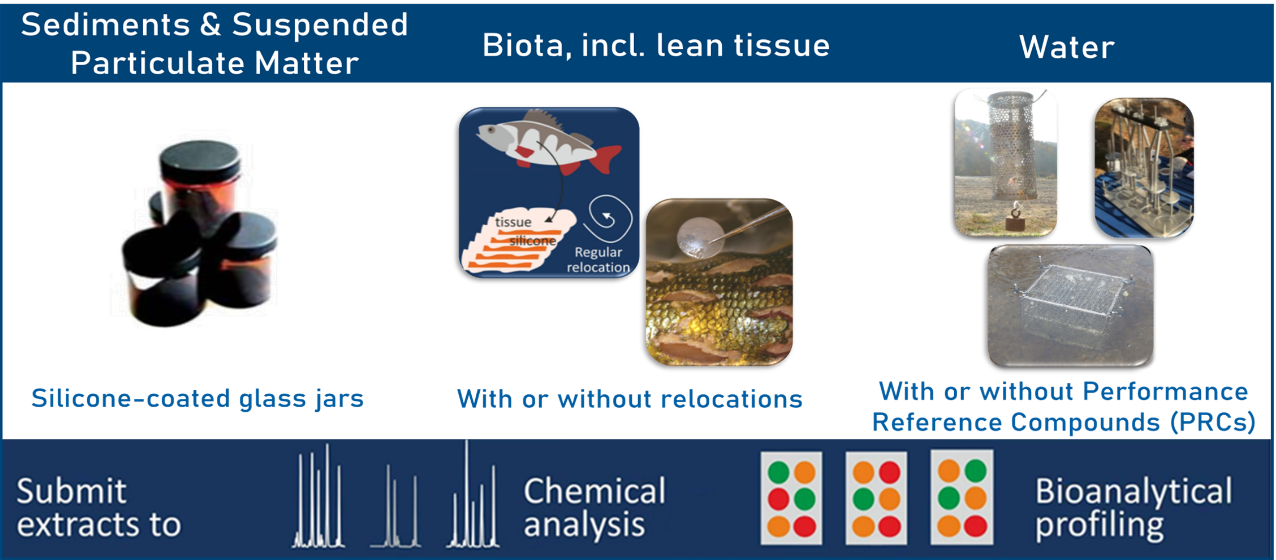
| Matrix | Non-polar organic chemicals | Polar organic chemicals |
|---|---|---|
| Biota | Silicone sheets | |
| Sediment/Soil/Suspended Particulate Matter | Silicone-coated jars | |
| Water |
Different silicone-based formats, other polymers, etc. |
Chemcatcher® |
Table 1: Specific PS expertise in our department
Suggestions for further reading
Mayer et al. 2003 (Feature Article introducing Passive Equilibrium Sampling Devices)
Rojo-Nieto et al. 2019 (passive equilibrium sampling of nonpolar compounds in biota)
Reiter et al. 2023 (passive equilibrium sampling of nonpolar compounds in different organs)
Römerscheid et al. 2023 (passive sampling of polar compounds in water)
Wernicke et al. 2022 (passive sampling with silicone in water and suspended particulate matter)
Charriau et al. 2016 (Chemcatcher review)
Vrana et al. 2001 (integrative passive sampling of nonpolar organics in water + theory)
Petersen et al. 2015 (Calibration of Chemcatcher® passive sampler for selected highly hydrophobic organic substances under fresh and sea water conditions)
Contact
Credits & History
Our expertise in the development and application of passive sampling technologies for monitoring of aquatic micropollutants was build up in the last 25 years esp. by our former senior scientist Albrecht Paschke in collaboration with Branislav Vrana (RECETOX, Brno, Czech Republic) and a considerable number of project partners and students.
For multimedia passive equilibrium sampling our fundamental work was initiated in close collaboration with Philipp Mayer (DTU, Kongens Lyngby, Denmark) and has been continued within the team of the ERC project CHEMO-RISK.
