Laboratories for Stable Isotopes (LSI)
Competence center for isotope analytics
The Laboratories for Stable Isotopes (LSI) are an association of UFZ research laboratories that build on a long-standing expertise in the analysis of stable isotopes (e.g., H, C, N, O, S, Cl), respective concepts and applications, and thus forming a unique center of competence in isotope analytics. We provide innovative, advanced instrumentation and laboratory facilities with expert guidance and support. The spectrum of available analytical techniques allows the bridging of scales from molecular levels up to catchment areas and is applied, for example, in the context of:
- monitoring of biogeochemical element cycles in relation to climate change adaptation, energy storage, and pollutants/nutrients transport and turnover in terrestrial and freshwater ecosystems
- forensic characterization of sources and sinks of chemicals (from production to removal and from legacy contaminants to micropollutants)
- estimating the source of organic matter and trophic position in food webs
- delineating water flow pathways and computing water and solutes travel times and age distributions
Service
Our core competencies are: (I) on-line bulk and compound-specific isotope analyses (BSIA and CSIA) and the respective laboratory sample preparation techniques, (II) method development for isotope analytics of gaseous, liquid and solid samples, (III) quality control and management for stable isotopes analysis, and (IV) the development and calibration of international isotope reference materials.
We welcome collaborations to expand and improve the applicability of stable isotope analytics. A list of available instrumentation and possible applications can be found below. If you have any questions regarding the application of stable isotopes in your experiments or the analysis of your samples, please contact us.
Available lab facilities and possible applications for isotope analytics
Questions to be addressed:
What is the origin of a compound?
What is the position of species in food webs?
How to reconstruct past environmental and climatic conditions?
How to authenticate food samples?
What is the isotopic ratio of a reference material used for compound-specific stable isotope analysis? ...
How to do:
Determine the C, N and/or S stable isotope ratio of a sample via elemental analyzer–isotope ratio mass spectrometry (EA-IRMS) or determine the H and/or O stable isotope ratio of a sample via high temperature conversion–isotope ratio mass spectrometry (HTC-IRMS).
Requirements for the sample:
The sample can be organic or inorganic, solid or liquid, at natural abundance level or isotopically enriched.
Examples from literature:
Organic reference materials for hydrogen, carbon, and nitrogen stable isotope-ratio measurements: Caffeines, n-alkanes, fatty acid methyl esters, glycines, l-valines, polyethylenes, and oils. Schimmelmann, A., Qi, H., Coplen, T.B., et al. Analytical Chemistry. 88, 4294-4302 (2016).
Multidimensional isotope analysis of carbon, hydrogen and oxygen as tool for identification of the origin of ibuprofen. Gilevska, T., Gehre, M. & Richnow, H.H. Journal of Pharmaceutical and Biomedical Analysis. 115, 410-417 (2015).
Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Post, D.M. Ecology 83, 703-718 (2002).
Contact:
Dr. Matthias Gehre (Leipzig)
matthias.gehre@ufz.de
+ 49 (0) 341 6025 1361
Dr. Kay Knöller (Halle/Saale)
kay.knoeller@ufz.de
+49 (0) 341 6025 4444
Dr. Steffen Kümmel (Leipzig)
steffen.kuemmel@ufz.de
+ 49 (0) 341 6025 1362
Dr. Mario Brauns (Magdeburg)
mario.brauns@ufz.de
+49 (0) 341 6025 4140
Questions to be addressed:
How to visualize dynamic processes (e.g., (bio)degradation of contaminants)?
How to differentiate processes which are acting on the same compound?
How to quantify (bio)transformation processes? ...
How to do:
Determine the C, N and/or H stable isotope ratios of a sample via gas chromatography–isotope ratio mass spectrometry (GC-IRMS) or determine the Cl and/or S (δ34S, δ33S) stable isotope ratios of a sample via gas chromatography–multiple-collector–inductively coupled plasma mass spectrometry (GC-MC-ICPMS).
Requirements for the sample:
The sample must be organic, but can be liquid or gaseous, at natural abundance level or isotopically enriched.
Examples from literature:
Investigation of active site amino acid influence on carbon and chlorine isotope fractionation during reductive dechlorination. Phillips, E., Bulka, O., Picott, K., et al. FEMS Microbiology Ecology. 98, fiac072 (2022).
Photosynthesis-driven methane production in oxic lake water as an important contributor to methane emission. Günthel, M., Klawonn, I., Woodhouse, J. et al. Limnology and Oceanography. 65, 2853-2865 (2020).
Distinct carbon isotope fractionation signatures during biotic and abiotic reductive transformation of chlordecone. Chevallier, M.L., Cooper, M., Kümmel, S., et al. Environmental Science and Technology. 52, 3615-3624 (2018).
Triple-element compound-specific stable isotope analysis of 1,2-dichloroethane for characterization of the underlying dehalogenation reaction in two Dehalococcoides mccartyi strains. Franke, S., Lihl, C., Renpenning, J., et al. FEMS Microbiology Ecology. 93 fix137 (2017).
Contact:
Dr. Matthias Gehre (Leipzig)
matthias.gehre@ufz.de
+ 49 (0) 341 6025 1361
Dr. Steffen Kümmel (Leipzig)
steffen.kuemmel@ufz.de
+ 49 (0) 341 6025 1362
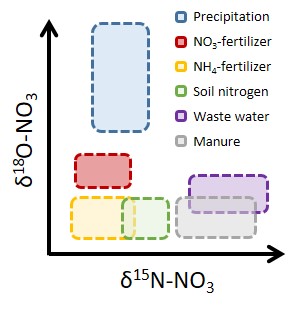
Questions to be addressed:
What sources contribute to a solute pool?
What physical and (bio)chemical processes affect the solute’s pool (e.g., dilution, mixing, natural or stimulated bacterial turnover…)?
What is the absolute amount and rate of solute turnover?...
How to do:
Determine the C, N, O and/or S stable isotope ratios of solutes in a sample via a sequence of chemical preparation and isotope analytical techniques (i.e. gas isotope ratio mass spectrometry, elemental analysis, high-temperature pyrolysis, off-axis integrated cavity output spectroscopy, denitrifier method).
Requirements for the sample:
The water or leachable solid sample must contain a sufficient amount of the target solute (e.g., nitrate, nitrite, ammonium, sulfate, phosphate, bicarbonate).
Examples from literature:
New Ag3PO4 comparison material for stable oxygen isotope analysis. Watzinger, A., Schott, K., Hood‐Nowotny, R., et al. Rapid Communications in Mass Spectrometry. 35, e9101 (2021).
High spatial-resolution monitoring to investigate nitrate export and its drivers in a mesoscale river catchment along an anthropogenic land-cover gradient.
Bujak, I., Müller, C., Merz, R., et al. Hydrological Processes. 35, e14361 (2021).
Tomography of anthropogenic nitrate contribution along a mesoscale river. Müller, C., Musolff, A., Strachauer, U., et al. Science of the Total Environment. 615 (2018).
Contact:
Dr. Kay Knöller (Halle/Saale)
kay.knoeller@ufz.de
+49 (0) 341 6025 4444
Dr. Christin Müller (Halle/Saale)
christin.mueller@ufz.de
+ 49 (0) 341 6025 4235
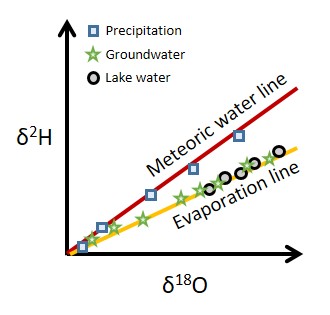
Questions to be answered:
Where does water originate?
What are the prevailing movement pathways of water and water vapor in aquatic and atmospheric systems across scales?
How do different compartments in aquatic systems interact?
How do extreme hydrologic events impact water and solute flow paths and travel times?...
How to do:
Determine the O and/or H stable isotope ratios of a water sample via laser-cavity ringdown spectroscopy.
Use high-frequency automated samplers specified for stable isotope sample collection.
Requirements for the sample:
The water sample must have a minimum volume of 5 mL and must be filtered. The sample can be taken manually or by a high-frequency autosampler.
Examples from literature:
Upscaling Tracer-Aided Ecohydrological Modeling to Larger Catchments: Implications for Process Representation and Heterogeneity in Landscape Organization Yang, X., Tetzlaff, D., Müller, C., et al. Water Resources Research. 59, e2022WR033033 (2023).
Technical note: A microcontroller-based automatic rain sampler for stable isotope studies. Michelsen, N., Laube, G., Friesen, J., et al. Hydrology and Earth System Sciences. 23, 2637-2645 (2019).
Contact:
Dr. Kay Knöller (Halle/Saale)
kay.knoeller@ufz.de
+ 49 (0) 341 6025 4444
Christina Radtke (Haale/Saale)
christina.radtke@ufz.de
+ 49 (0) 341 6025 4192
Dr. Christin Müller (Halle/Saale)
christin.mueller@ufz.de
+ 49 (0) 341 6025 4235
Information on applications of stable isotope analytics in natural sciences:
Isotopic composition of nitrogen species in groundwater under agricultural areas: A review. Nikolenko, O., Jurado, A., Borges, A.V., et al. Science of the Total Environment. 621, 1415-1432 (2018).
On the use of stable isotopes in trophic ecology. Boecklen, W.J., Yarnes, C.T., Cook, B., et al. Annual Review of Ecology, Evolution, and Systematics. 42:1, 411-440 (2011).
Principles and Mechanisms of Isotope Fractionation. Hunkeler, D. & Elsner, M. In Environmental Isotopes in Biodegradation and Bioremediation, Taylor & Francis Group, Boca Raton, FL (2010).
Information on instrumentation for on-line bulk and compound-specific stable isotope analysis:
Perspectives of compound-specific isotope analysis of organic contaminants for assessing environmental fate and managing chemical pollution Hofstetter, T.B., Bakkour, R., Buchner, D., et al. Nature Water. 2, 14-30 (2023).
A review on environmental isotope analysis of aquatic micropollutants: Recent advances, pitfalls and perspectives. Blessing, M. & Baran, N. Trends in Analytical Chemistry. 157, 116730 (2022).
Recent advances in multi-element compound-specific stable isotope analysis of organohalides: Achievements, challenges and prospects for assessing environmental sources and transformation. Nijenhuis, I., Renpenning, J., Kümmel, S., et al. Trends in Environmental Analytical Chemistry. 11, 1–8 (2016).
Networks and activities
The biannual UFZ - Isotope platform meeting intends to bring together current and potential future users of stable isotope analytics. A central element of the meeting are presentations of new possibilities and applications of stable isotope analytics in the context of the UFZ research. Additionally, future needs and interests of UFZ researchers can be discussed.
Please contact us if you are interested in participating the Isotope Network Meeting.
Contact:
Dr. Steffen Kümmel
steffen.kuemmel@ufz.de
+ 49 (0) 341 6025 1362
We offer a course that provides an overview of stable isotope concepts. This ranges from assessment of biogeochemical processes including the uptake, transport and transformation of chemicals in microbial cultures, in complex microbial communities (including cell-to-cell interactions) to environmental processes at the catchment scale. The two main principle approaches using natural abundance or stable isotope tracers are introduced with specific concepts, equipment involved and examples of applications. Additionally, specialized courses are currently being considered.
Please contact us if you are interested in taking course on stable isotope concepts or methods.
Contact:
Dr. Ivonne Nijenhuis
ivonne.nijenhuis@ufz.de
+ 49 (0) 341 6025 1356
The LSI cooperates with multiple national and international partners. The expertise of the LSI is applied in a large variety of process studies as well as in projects for quality control and quality assurance in stable isotope analysis in cooperation with:
International Atomic Energy Agency (IAEA)
National Institute for Standards and Technology (NIST)