IP4 Science Talks
'CyanoCapture: is it possible to turn CO2 into $40bn worth of drug molecules?'

Dr. David Kim
Magdalen Centre, The Oxford Science Park
16.10.25, 09:30 am in Building 7.3, R7.013
Abstract
IP4 Science Talks
'Hydrogenase Superassemblies Orchestrate CO₂ Fixation at the Thermodynamic Limit of Life'

Dr. Jan Schuller
Philipps Universität Marburg
13.11.25, 10:30 am in Building 1.0, Room 7.013A+B
Abstract
Using a scale-bridging approach that combines genetics in non-model organisms, biochemical assays, single-particle cryo-EM, and cryo-electron tomography, we reveal how WLP hydrogenases assemble into supramolecular structures of remarkable scale and complexity. In acetogens, the hydrogen-dependent CO₂ reductase (HDCR) forms polyferredoxin-decorated nanowires that accelerate electron flow, underpinning the exceptionally rapid conversion of H₂ and CO₂ into formate. In methanogens, Hdr–Vhu–Fwd hydrogenase complexes assemble into 8 MDa circular electron nanowires, in which polyferredoxin subunits directly couple electron bifurcation to CO₂ fixation in the methyl branch of methanogenesis.
The recurring use of polyferredoxin scaffolds in these hydrogenase assemblies reveals a conserved principle by which life sustains metabolism at the thermodynamic limits of energy availability. More broadly, the formation of hydrogenase superassemblies illustrates a general strategy by which organisms maximize catalytic efficiency and energy conservation under extreme conditions, providing a conceptual framework for how ancient life thrived and offering blueprints for bioinspired strategies for sustainable CO₂ utilization.
IP4 Science Talks
'Decoding and Taming Microbial Nitrogenases for CO2 Conversion'

Dr. Johannes Reberlein
Max Planck Institute for Terrestrial Microbiology
04.09.25, 01:00 pm in Building 1.0, Room 254
Key Words: Nitrogenase, N2 fixation, CO2 Reduction
Abstract: Nitrogenases are the only known enzymes that catalyze the reduction of molecular nitrogen (N2) to ammonia (NH3). The N2 reduction drives the biological nitrogen fixation and the global nitrogen cycle. Besides the conversion of N2, nitrogenases were recently shown to convert carbon dioxide (CO2) to carbon monoxide, formate and hydrocarbons,(1-5) suggesting CO2 to be a competitor of N2 (Fig. 1). However, the impact of omnipresent CO2 on N2 fixation has not been investigated to date.We comprehensively studied the competing reduction of CO2 and N2 by the two nitrogenases of Rhodobacter capsulatus, the molybdenum (Mo)- and the iron (Fe)-nitrogenase in vitro and in vivo (Fig. 1).(5) We identified significant differences among the Mo- and Fe-nitrogenase systems.(5) In vitro, the Fe-nitrogenase is almost threefold more efficient in CO2 reduction and profoundly less selective for N2 than the Mo-isoform under mixtures of N2 and CO2. Also in vivo, inside R. capsulatus the Fe-nitrogenase reduces CO2 readily into formate and methane, under physiological CO2 concentrations and in the presence of the main substrate N2. The in vivo CO2 activity of the Fe-nitrogenase facilitates the light-driven accumulation of formate and methane, one-carbon substrates for other microbes, and feedstock chemicals for a circular (bio)economy.
References:
1. Rebelein, J. G., Hu, Y., and Ribbe, M. W. (2014) Differential reduction of CO(2) by molybdenum and vanadium nitrogenases Angew Chem Int Ed 53, 11543-11546 10.1002/anie.201406863
2. Rebelein, J. G., Hu, Y., and Ribbe, M. W. (2015) Widening the Product Profile of Carbon Dioxide Reduction by Vanadium Nitrogenase ChemBioChem 16, 1993-1996 10.1002/cbic.201500305
3. Rebelein, J. G., Stiebritz, M. T., Lee, C. C., and Hu, Y. (2017) Activation and reduction of carbon dioxide by nitrogenase iron proteins Nat Chem Biol 13, 147-149 10.1038/nchembio.2245
4. Oehlmann, N. N., and Rebelein, J. G. (2022) The Conversion of Carbon Monoxide and Carbon Dioxide by Nitrogenases ChemBioChem 23, e202100453 10.1002/cbic.202100453
5. Oehlmann, N. N., Schmidt, F. V., Herzog, M., Goldman, A. L., and Rebelein, J. G. (2024) The iron nitrogenase reduces carbon dioxide to formate and methane under physiological conditions: A route to feedstock chemicals Sci Adv 10, eado7729 10.1126/sciadv.ado7729
IP4 Science Talks
'Semicon soft porous interfaces and GIWAXS-based operando'

Prof. Mingshui Yao
Institute of Process EngineeringChinese Academy of Sciences, Beijing
27.06.25, 09:30 am in Building 7.3, Room 7.013
Abstract
Some crystalline porous materials (CPMs), especially metal-organic frameworks (MOFs) or porous coordination polymers (PCPs), exhibit soft porosity arising from their lattice flexibility (named soft porous crystals), which has garnered significant attention owing to their potential to switch functionalities [1, 2]. Such crystals enable the integrated “sieving-enrichment-recognition-response” strategy to address the dandling challenge of accurate detection of trace-level gas molecules in complex systems. However, the lack of clarity regarding the mechanisms of high-quality nano-micro structures formation and interface regulation in molecule-based materials constrains their developments in high-performance device integrations and practical applications [3].
To explore the semicon soft porous interfaces, we reported that:
1) Interparticle growth: a strategy to grow 'face-on' and 'edge-on' cMOF nanofilms on substrates by controlling the “stand-up” behaviors of ligands on various surfaces to overcome the difficulty in the orientation control of such film [4], and the establishment of operando methodology, thereby demonstrating the softness of the crystalline nanofilms and revealing their unique anisotropic softness–dependent conductive functions.
2) Intraparticle modulation: the modulated conductivity and topology of cMOFs by incorporating electron-donating fused thiophene rings into the frameworks and extending their π-conjugated systems through in-situ ring-closing reactions [5]. The inherent tunable electrical properties and topology significantly affect the charge transport efficiency and gas diffusion of NH3, respectively, and thus modulate the sensing properties of the corresponding chemiresistive gas sensors.
3) Hybridization: the molecular-level integration of insulating iMOFs and conductive cMOFs (cMOF-on-iMOF) heterostructures to realize direct electrical control [6]. The enhanced gate-type selective sorption of CO2, even without electrical control, confirms the existence of a porous interface. Under direct electrical control and the transduced electrical signals, the cMOF-on-iMOF heterostructures with the semiconductive soft porous interfaces exhibit visible flexibility and electrical “shape memory” effects, as revealed by operando synchrotron measurements.
4) Hybridization: the energy-level structural engineering of MO@MOF (MO=metal oxide) heterojunctions to optimize chemiresistive sensing performance [7]. The interface was flexibly modulated from straddling gap to staggered gap type by manipulating the –NH2 functionalization of TiO2@(NH2)x-MIL-125 from x = 0 to 1 or 2, respectively. As a result, TiO2@NH2-MIL-125 is the first light-activated material to detect NO2 at 1 ppb at RT.
References
[1] M.-S. Yao, J. W. Xiu, G. Xu* et al. Angew. Chem. Int. Ed. 2019, 58, 14915-14919.
[2] S. Horike*, S. Kitagawa*. Nat. Mater. 2022, 21(9): 983-985.
[3] M.-S. Yao, K.-i. Otake*, S. Kitagawa*. Trends Chem. 2023, 5(8), 588-604.
[4] M.-S. Yao, K.-i. Otake,* S. Kitagawa* et al. Proceedings of the National Academy of Sciences (PNAS). 2023, 120 (40) e2305125120.
[5] M.-S. Yao,* Rui Wang,* Gen Zhang* et al. Angew. Chem. Int. Ed. 2024, 63(16), e202401679.
[6] M.-S. Yao, K.-i. Otake*, S. Kitagawa* et al. Angew. Chem. Int. Ed. 2023, 62 (35): e20230390.
[7] C. Li*, M.-S. Yao*, G. Xu* et al. Angew. Chem. Int. Ed. 2024, DOI: 10.1002/anie.202419195.
CV of the presenter
Ming-Shui Yao currently works at the Institute of Process Engineering (IPE), CAS, China as a full professor, PI, and serves as the Deputy Director of the State Key Lab of Mesoscience and Engineering. He received his Ph.D. in Materials Science from IPE, CAS. Then he worked at FJIRSM, CAS, China (with Prof. Gang Xu), Kyoto University, Japan, as a JSPS fellow (with Prof. Susumu Kitagawa), and University of Birmingham, UK, as a Marie Skłodowska–Curie fellow (with Prof. Shangfeng Du, Prof. Robert Steinberger-Wilckens). He was awarded the “Excellent Young Scientists (Overseas)” by the National Natural Science of China (2022), and the “Young Particuology Award” by the Chinese Society of Particuology (2024). He does research in the interface chemistry of soft porous crystals and their applications in electrical devices, water treatments and gas-handling operations. As the first or corresponding author, he published over 30 SCI papers (over 80 in total, 6 ESI highly cited papers) in international peer-reviewed journals of chemistry and materials such as Angew. Chem., PNAS, Adv. Mater., Nat. Sci. Rev. etc. He is the Young Star Editor or editorial board member of Nano Research, Nano-Micro Letters, Chinese Journal of Chemistry and Chinese Journal of Structural Chemistry. He is very active in academic activities and has a team of 2 associate professors, 2 assistant professors, 1 research associate, and 9 students
IP4 Science Talks
'PROMICON final annual meeting'

Presentation of final results to the interested public
Scientists of PROMICON
30.04.25, 09:00 am till 01:00 pm in KUBUS 1CD
2: Juan Lopez Galvez: A double-staining automated flow cytometry method for real-time monitoring of bacteria in continuous bioreactors
3: Matina Roussou: Boosting photosynthetic acetate production through enhanced carbon flux in Cyanobacteria
4: Jose Pinto: Hybrid AI/Physical controller of natural microbiomes: Physical Informed Neural Networks (PINNs) for tight microbial control
5: Hannah Berreth: Hydrogen production in Rhodopseudomonas palustris dominated mixed biofilm consortia
6: Caroline Ruhl: The weird and wonderful metabolism of cyanobacteria!
IP4 Career Talks
'Being a Biologist in a Chemical Company: White Biotechnology at BASF & Career Opportunities'

Dr. Andrea Herold
BASF White Biotechnology Research
Group Leader Microbial Processes
29.04.25, 04:00 pm in KUBUS 2AB
IP4 Science Talks
'Statistical workflows for microbial data analysis - a guided tour from large-scale community analysis to single cells'

Prof. Christian L. Müller
Biomedical Statistics and Data ScienceLudwig-Maximilians-Universität, München
10.03.25, 1 pm in Building 4.0, Room 101
Abstract
In this talk, I will give an overview of some of the statistical methods and workflows that we have been developing in the past decade, spanning the analysis of large-scale microbiome survey data, synthetically designed microbial communities, all the way down to single species growth, single cell RNA-Seq, and, more recently, flow cytometry measurements. Using different habitats and measurement technologies, I will illustrate several statistical methods and workflows that may be of general interest for the analysis of microbial data. This will include learning statistically sound large-scale association networks from microbiome relative abundance data, predictive modelling of function or environmental covariates from microbial community abundance data, differential microbial growth analysis of large-scale chemical perturbation screens, and statistical analysis of bacterial single-cell assays. The latter will include new statistical tools for single-cell RNA-Seq data as well as single-cell flow cytometry measurements. I will keep mathematical aspects of our workflows to a minimum, focusing more on the research questions one can answer with the methods, and which one cannot.
IP4 Science Talks
'Exoelectrogenesis as an enduring extracellular electron transport pathway in photosynthetic bacteria '

Dr. Laura Wey
University of Turku, Finnland
03.12.24, 2:30 pm in KUBUS 1CD
Abstract
IP4 Science Talks and MEB
'Design meets evolution: Theory and practice'

Prof. Victor de Lorenzo
Centro Nacional de Biotecnología, CSIC
28.10.24, 1:30 pm in KUBUS 2AB
Talk is part of the Pseudomonas Grassroots Meeting but open to all interested people.
Abstract
IP4 Science Talks and MEB
'Biodegradation of antibiotic pollutants –
A way to tackle the spreading of ARGs in One Health context?'

Prof. Philippe Corvini
Group Leader Environmental Biotechnology
03.09.24, 2:30 pm in KUBUS 1CD (new room!)
Abstract
Sulfonamides are the second most used antibiotics worldwide with a release of approximately 20,000 tons per year. Sulfamethoxazole (SMX) is often detected in environmental compartments. The spreading of antibiotic resistant bacteria and antibiotic resistant genes is of concern and shall be integrated in One Health approaches.
Procedure/method
Microbacterium BR1 was isolated from a membrane bioreactor. Mutants defective for sul1 and/or sadA were obtained by sub-culturing Microbacterium BR1. Degradation experiments were carried out using growing and resting cells with SMX and 14C-labelled SMX. Metabolites were analyzed using LC-MS. Genomic data was acquired by Oxford nanopore and Illumina sequencing. The proteome was analyzed using 2D-gel electrophoresis and MS. The number of copies of sadA was determined using qPCR. Transcriptomic analyses were performed.
Findings/results
Microbacterium BR1 can feed on SMX as sole carbon and energy source. The degradation of SMX starts with an ipso-substitution, catalyzed by a flavin-dependent monooxygenase (SadA) acting with a FMN reductase (SadC). The resulting p-aminophenol enters the central metabolism through a second monooxygenase activity (SadB), which leads to products amenable to ring opening. The cluster of degradation genes was identified and each of these three enzymes (SadA, SadB and SadC) was expressed in E. coli. The resistance gene sul1 was also detected in Microbacterium BR1.
We discuss the relevance of these findings by addressing questions arising from this research. Finally, we isolated mutants defective for sul1, sadA and the double mutant lacking both genes. We demonstrated that the sadA gene is a novel sulfonamide resistance gene. We conclude that the sul1 mutant is even more resistant to SMX than the wild type.
Implications/Applications
We demonstrate that the gene sadA of the cluster responsible for sulfonamide degradation confers sulfonamide antibiotic resistance to Microbacteriaceae. This fact and another evidence, i.e. gene cluster flanked by relaxase encoding sequences point out to possible mobility of these genes and necessitate further works to elucidate whether sadA can be subject to horizontal gene transfer.
IP4 Science Talks and AG Ecothermodynamics/Biocalorimetry
'The energetic cost of lipid remodeling by minimal cells'

Prof. Karim Fahmy
Institute of Resource Ecology
26.08.24, 2:30 pm in KUBUS 1CD
Abstract
1. Breuer, M. et al. “Essential metabolism for a minimal cell“ Elife, 2019, 8, e36842.
2. Fahmy, K. “Simple Growth-Metabolism Relations are revealed by conserved patterns of heat flow from cultured microorganisms” Microorganisms, 2022, 10, 1397.
Short CV
Karim Fahmy received his PhD at the University of Freiburg in membrane protein research. During his postdoc studies as a Howard Hughes Medical Institute Fellow with Prof. Tom Sakmar at Rockefeller University, New York, he performed seminal work in combining site-directed mutagenesis with infrared spectroscopy to elucidate functional mechanisms of G protein-coupled receptors. After his habilitation at the University of Freiburg he joined the Helmholtz-Zentrum Dresden-Rossendorf heading the Department of Biophysics. His lab investigates and develops synthetic membrane mimetics to reveal the influence of the lipidic phase on membrane protein function. In addition to various spectroscopies, the lab uses calorimetric methods to investigate both biomolecular interactions as well as the systems biology of lipids in metabolic processes of synthetic minimal cells. Novel evaluation methods for microcalorimetric data are developed based on these simplest organisms. Karim Fahmy is affiliated Professor at the University of Dresden. He has headed the Molecular Biophysics Section of the German Biophysical Society and is recipient of the Hans-Griesebach-Price of the University of Freiburg.
IP4 Science Talks and TECH
'PFAS interactions with ion-exchange materials'

Prof. Lee Blaney
Professor of Chemical, Biochemical, and Environmental Engineering
University of Maryland Baltimore County (UMBC), USA
01.08.24, 10:30 am in KUBUS 2AB
Abstract
Short CV
Lee received his BS and MS degrees in Environmental Engineering from Lehigh University. After completing his PhD in Civil Engineering at the University of Texas at Austin, he started as an Assistant Professor of Chemical, Biochemical, and Environmental Engineering at the University of Maryland Baltimore County, where he is now Professor. Lee also serves as President-Elect of the Association of Environmental Engineering and Science Professors (AEESP) and Executive Editor at the Journal of Hazardous Materials. He is the recipient of the ES&T James J. Morgan Early Career Award, the National Science Foundation Career Award, and the AEESP Award for Outstanding Teaching in Environmental Engineering and Science.
IP4 Science Talks and AG Biophotovoltaics
'Engineering Pseudomonas as an universal platform for detection of chemicals'

Prof. Pablo Nikel
Systems Environmental Biology
DTU Biosustain, Denmark
06.05.24, 09:30 am in KUBUS 1CD
Abstract
Short CV
Prof. Pablo I. Nikel earned a Ph.D. in Biotechnology and Molecular Biology (2009) in Argentina. After working in the US (Rice University, supported by the ASM), Pablo moved to Europe as a post-doctoral fellow, funded by the European Molecular Biology Organization (EMBO) and the MSCA of the European Commission, working in the laboratory of Prof. Víctor de Lorenzo (Madrid). Since 2017, he has been leading the Systems Environmental Microbiology Group at DTU Biosustain. Pablo’s team aims at rewriting Pseudomonas putida’s core biochemistry through synthetic metabolism for biosynthesis of novel compounds with a focus on new-to-nature fine chemicals (www.sem-cfb.com). The ambition of this research programme is to expand the limits of microbial biochemistry by engineering P. putida to access compounds that, as of today, are exclusively produced via traditional chemistry nowadays. Pablo is Full Professor of the Technical University of Denmark (since 2023) and, among other obligations, he is Editor-in-Chief of Current Opinion in Biotechnology and a cofounder of BioHalo, a spin-out company from his laboratory pursuing biological production of fluorochemicals.
IP4 Science Talks and MIBITECH
'Electroactive biofilms and their applications for resource recovery'

Prof. Annemiek ter Heijne
Environmental Technology
Wageningen University & Research
03.04.24, 2:30 pm in KUBUS 1A
Abstract
Short CV
Annemiek ter Heijne is professor in (Bio)Recovery Technology for Circular Economy at the Environmental Technology group of Wageningen University & Research (The Netherlands). She obtained her PhD degree on Microbial Fuel Cells and has continued her career in Wageningen working on different applications that involve the interaction between microorganisms and electrodes. She has been board member and president of ISMET, the International Society of Microbial Electrochemistry & Technology, and her teaching focuses on Renewable Energy Technologies and Microbial Electrochemistry. In her free time, she loves to spend time with her family, she likes spending time outdoor (hiking and running), and cooking (vegan) food.
IP4 Science Talks
'Tuning multi-enzyme catalysed processes – modular cascade set-up, activity regulation, and integration with microbial and chemical transformations'

Prof. Dörte Rother
Systems Biotechnology, AG Synthetic Enzyme Cascades
Forschungszentrum Jülich
28.02.24, 2:30 pm in KUBUS 1CD
Abstract
countries). The establishment of more biocatalytic steps in chemical syntheses is one possible solution, as enzymes and whole cells offer sustainable advantages, such as biodegradability, intoxicity, high selectivity, and many more. As a myriad of enzymatic reactions exist for almost any product, their potential is immense. Great scientific achievements and new techniques have enabled the design of economically and ecologically feasible one-step and multi-step enzyme catalysed reactions. This presentation will highlight the advantages of multi enzyme catalysed processes especially with respect to atom- and step efficiency, selectivity and modularity. However, with new opportunities, also new challenges arise. Two new possibilities, including our efforts to circumvent the associated disadvantages, will be subsequently presented: (A) avoiding cross-reactivity in (self-) regulated one-enzyme cascades and (B) the potential of using renewable resources as starting materials when microbial cell factories, enzymes and chemical catalysts are combined effectively. (A) The more enzyme steps are combined, the higher the risk of undesired cross-reactivity. Separation in space or on time can solve this issue. In the ‘LightCas’-project we investigate the possibilities to avoid cross-reactivity in one-pot systems by separation of reaction steps in time. With the help of sequential enzyme addition and on-demand light-induced enzyme inactivation 1 , a tight control of each biocatalytic step in a one-pot cascade is possible. This results in high product purity. Due to online analytics and automation, our ultimate goal is in close reach: setting up a one-pot multi-step light-controlled enzyme reactor yielding tetrahydroisoquinolines2 from cheap substrates with high selectivity and concentration in a technically self-controlled manner. (B) The combination of suitable chemical and biological catalysts with their intrinsic advantages holds the potential to develop processes that are truly superior to current production methods in terms of efficiency and sustainability. In such hybrid processes, e.g. microorganisms can use substrates from human waste streams from agriculture and the food industry to provide simple chemical building blocks. Then, enzymes are used to diversify these compounds in vitro, enabling the construction of product platforms of valuable fine chemicals, complex (chiral) building blocks and active pharmaceutical ingredients. In addition, chemical transformations can complement and diversify this product portfolio. This approach will be shown on the examples of (i) a hybrid process for the bio-based production of chiral amino alcohols from xylose and glucose 3,4 (Figure 1) and (ii) the adaptive use of renewable raw materials and the integration of CO2 in the chemo-enzymatic production of chiral diols and dioxolanes. Challenges, like the identification of suitable reaction conditions (including unconventional media) for each individual catalysts and the need of integrating downstream processing 6 to make these hybrid processes truly advantageous over other strategies will be discussed.
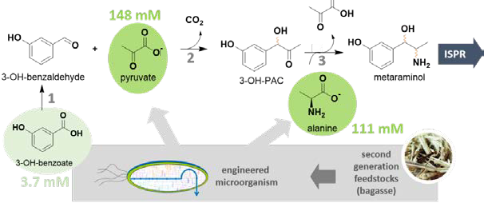
Figure 1. Enzymatic cascade towards metaraminol starting from renewables. The cascade comprises of a carboxylic acid reduction (1), a carboligation (2) and a transamination (3) step. The aliphatic precursors pyruvate and L -alanine can be produced microbially from second generation feedstocks (D-glucose, D-xylose) in suitable amounts. In situ product removal (ISPR) is used to shift the reaction equilibrium of the transamination step.
Short CV
Dörte Rother holds a diploma degree in biology from RWTH Aachen University. She conducted her PhD at the Heinrich-Heine University Düsseldorf (with research stays at Karolinska Institute Stockholm, University of Freiburg and University of Stuttgart). In 2008 she received a DFG-postdoc scholarship to work at Aachen University and became in 2009 postdoctoral student at the IBG-1: Biotechnology at Research Center Jülich. In 2012 she started her own Helmholtz-young investigators group in Jülich, additionally funded by a ERC-starting grant in 2017. In 2018 Dörte Rother was appointed as full professor for `synthetic enzyme cascades’ at Aachen University and leads the group `biocatalysis’ at Research Center Jülich. She has three children. She received the DECHEMA-Prize 2018 and the Biotrans Junior Award 2019 for her outstanding research activities in multi-step biocatalysis and sustainable process development.
IP4 Science Talks
'Closing cycles in the plastics bioeconomy through Pseudomonas biotechnology'

Prof. Nick Wiercx
Microbial Catalysis
Forschungszentrum Jülich
31.01.24, 2:30 pm in KUBUS, 1A
Abstract
In this context, we see great potential in the bio-upcycling of plastic waste, especially when combined with the development of new polymers and composites tailored for end-of-life functionalities such as biodegradability. Such polymers often contain biosimilar C-O and C-N heteroatom linkages, including polyesters, polyamides, and polyurethanes (1). Upon enzymatic or chemical hydrolysis of such polymers, a mixture of monomers is released containing alcohol, acid, or amine groups. Depending on the starting substrate the separation of these mixtures into their individual constituents may not be economical. In this case, we propose to use these plastic hydrolysates as carbon source for biotechnology (2). To enable this, we have developed Pseudomonas into a plastic monomer-metabolizing biotechnological workhorse, capable of metabolizing a wide range of aliphatic diols, dicarboxylates, and amines. Currently our main focus is to develop these monomer-metabolizing strains to not just grow on plastic monomers, but to convert plastic hydrolysates into value-added chemicals and biopolymers.
Besides addressing the end-of-life of plastics, it is also important to address their beginning. Currently plastics are primarily fossil-based which ultimately causes climate change, petrochemical pollution, and a dependence on politically unstable countries with poor humanitarian records. We therefore also develop Pseudomonas catalysts for the bio-based production of chemicals, including plastic building blocks. Both aspects will be discussed in the context of transitioning plastics into the circular bioeconomy.
(1) Ellis et al. (2021) Chemical and biological catalysis for plastics recycling and upcycling. Nature Catalysis 4:539-556
(2) Tiso et al. (2022) The metabolic potential of plastics as biotechnological carbon sources – Review and targets for the future. Metabolic Engineering 71:77-98
IP4 Science Talks and SOMA
'Cyanobacterial growth and the productivity of phototrophic cultures: insights from computational models'

Dr. Ralf Steuer
Institute for Theoretical Biology
Humboldt-University Berlin
29.11.23, 3:00 pm in Building 4.0, Room 101
Abstract
The evolution of oxygenic photosynthesis in the ancestors of modern-day cyanobacteria gave rise to perhaps the most important biological process within our biosphere: the assimilation of atmospheric CO2 into organic biomass and the release of molecular oxygen as a byproduct. While properties of cyanobacterial growth in axenic cultures are increasingly understood, there are still many open questions regarding the limits of cyanobacterial growth. In particular, the economic viability of cyanobacterial biotechnology is still constrained by low growth rates and low areal productivities.
In the talk, I will discuss computational models of cyanobacterial metabolism and growth, from genome-scale reconstructions to coarse-grained kinetic models of growth. A particular focus will be optimality and resource allocation. That is, how do microbial and, in particular, cyanobacterial cells allocate their finite cellular resources, such as a finite proteome or a finite cytosolic space, to ensure maximal evolutionary success in a given environment. A second theme will concern the implications of resource allocation for cyanobacterial productivity. It is shown that high maximal growth rates are not a sufficient or necessary property for high phototrophic productivity. Finally, I will discuss how the perspective from (optimal) resource allocation can help us to understand the evolution of interactions between phototrophic and heterotrophic microbes as part of microbial ecosystems.
Short CV
Ralf Steuer studied Physics in Munich and Berlin. Previous works include time-series analysis, information theory, analysis of metabolomic data, and computational model of cellular metabolism. Within the past 10 years a particular focus has been on computational descriptions of cyanobacterial metabolism and growth. The research group published genome-scale metabolic reconstructions of several cyanobacterial strains and pioneered the use of large-scale models of time-dependent resource-allocation in phototrophic microorganisms.
References
[1] Bruggeman, FJ, Teusink, B, Steuer, R (2023). Trade-offs between the instantaneous growth rate and long-term fitness: Consequences for microbial physiology and predictive computational models. BioEssays, 45, e2300015.
[2] Faizi M, Steuer, R (2019) Optimal proteome allocation strategies for phototrophic growth in a light-limited chemostat. Microb Cell Fact 18, 165
[3] Zavřel T, Faizi M, et al. (2019) Quantitative insights into the cyanobacterial cell economy. eLife 8:e42508
Lecture of AGs Plant Biogeochemistry and Ecothermodynamics/Biocalorimetry
'Cadmium in cacao crop: Using bioremediation as lead strategy in Colombia'

IP4 Science Talks
'Chemoproteomics to Identify Metabolite Regulation of Photosynthesis and Carbon Fixations'

Prof. Paul Hudson
School of Biotechnology
KTH Royal institute of Technology Stockholm, Sweden
Metabolic engineering of microbes to produce compounds of interest often involves dramatic alteration of metabolite pools. An often-overlooked consequence is that accumulating metabolites may negatively affect enzyme activity through competitive or allosteric inhibition. Additionally, post-translational regulation of enzyme activity can complicate metabolic engineering, as pathway flux becomes less sensitive to artificial enzyme overexpression. Here I will describe our adaptation of two chemoproteomics methods to identify metabolite-protein interactions in the proteomes of cyanobacteria and chloroplasts. These techniques rely on changes in thermal stability or of proteolytic digestion susceptibility in the presence of an effector metabolite, and together allow us to map metabolite-protein binding surfaces. In an initial effort, we found widespread interaction of tested metabolites with enzymes in central carbon metabolism, but that only a fraction of these interactions affect catalysis. For example, the Calvin cycle intermediate glyceraldehyde phosphate (GAP) stimulated activity of the Calvin cycle enzyme F/SBPase in reducing conditions, representing a feed-forward activation of the cycle. In oxidizing conditions however, GAP inhibited the enzyme by promoting aggregation. I will also describe efforts in mutagenizing enzymes to reduce sensitivity to metabolite regulation, by high-throughput screening of the effects of enzyme mutation on both cell growth and product synthesis rates.
Paul Hudson is associate professor in the School of Biotechnology at KTH Royal Institute of Technology in Stockholm, Sweden. He studied chemical engineering at NC State and UC Berkeley, and did post-doctoral training at KTH on bacterial surface display (2012-2014). His research as a group leader is on metabolic engineering of CO2-fixing bacteria, with focus on Calvin cycle bacteria such as cyanobacteria. The research uses systems biology methods such as proteomics to study CO2 fixation metabolism, and to guide engineering strategies for enhancing CO2 uptake and conversion. His group also develops synthetic biology tools in these strains.
IP4 Science Talks
'Climate extremes alter controls and pathways of soil carbon loss from alpine floodplains'

Prof. Marco Keiluweit
Soil & Microbial Biogeochemistry
Institute of Earth Surface Dynamics at the University of Lausanne, Switzerland
28.06.23, 1 pm in KUBUS hall 1A
Floodplains within mountainous catchments are dynamic reservoirs of carbon and nutrients, and experience seasonal flooding due to snowmelt and drainage. Climate change is rapidly altering snowpack and melt dynamics, resulting in greater variability in flooding and droughts. The variable hydrology drives spatial and temporal redox gradients within floodplain soils, with largely unknown consequences for carbon storage in and export from such ecosystems. In this presentation, I will report on a high-resolution monitoring campaign across extremely low and high river discharge years, foreshadowing climate change predictions, in a headwater catchment in the Rocky Mountains (Colorado, US). Combining in-field biogeochemical measurements with molecular and geochemical measurements, I will show that such extreme hydrological extremes undermine mineral and metabolic constraints on soil carbon cycling, altering pathways and rates of carbon loss from mountainous floodplains. Global implications of our findings for predicting climate change impacts on carbon cycling within dynamic floodplains globally will be discussed.
Prof. Dr. Marco Keiluweit is interested in how biogeochemical mechanisms controlling carbon and nutrient cycles in soil and sediments respond to climate and land use change. He completed his PhD at Oregon State University, and worked as a postdoc at Stanford University and as Assistant Professor at the University of Massachusetts-Amherst. He is now Professor of Soil & Microbial Biogeochemistry in the Institute of Earth Surface Dynamics at the University of Lausanne, Switzerland. His research combines laboratory, greenhouse, and field experiments with advanced analytical tools such as synchrotron spectroscopy, chemical imaging, and molecular microbiology. His group’s work links fine-scale biogeochemical mechanisms to landscape-scale processes within natural and managed ecosystems. Prof. Keiluweit has acquired numerous grants and has received several prestigious awards, including US DOE Lawrence Scholar and NSF Early Career awards.
TUCHEM Lecture
'Emerging Two-Dimensional Materials for Environmental Applications'

Dr. Ali Shaygan Nia
Professur für Molekulare Funktionsmaterialien
TU Dresden
Among different synthetic protocols, electrochemical exfoliation [1] of layered materials is a very promising approach that offers high yield, great efficiency, low cost, simple instrumentation, and excellent up‐scalability. Remarkably, playing with electrochemical parameters enables functionali-zation and tunable material properties and increases the material diversities from graphene to a broad spectrum of 2D semiconductors [2].
High solution processability of 2D materials via electrochemistry also offers hybridization/ functionalization of 2D materials and improve their processabilities into functionalized inks/paste/formulations. Wet chemistry also enables us to develop transition metal carbides/nitrides (Mxene) via safe and sustainable flouride-free approaches.
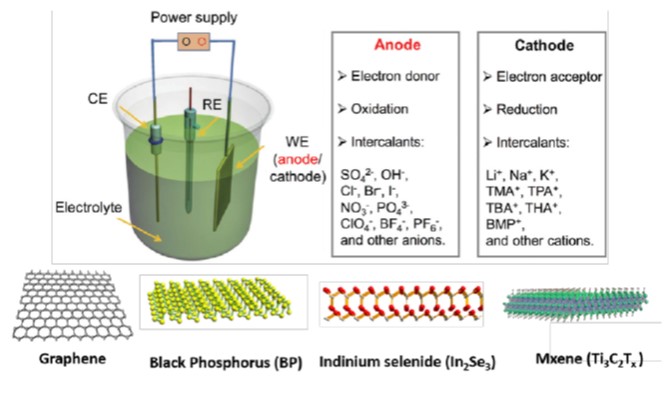 Figure 1. Schematic illustration of electrochemical exfoliation methodology and different 2D
Figure 1. Schematic illustration of electrochemical exfoliation methodology and different 2D
materials which could be produced via this method
References:
[1] Adv. Mater. 2020, 32, 1907857.
[2] Adv. Mater. 2020, 32, 1907244; Small 2019, 15, 1901265; Angew. Chem. Int. Ed. 2018, 57, 4677-4681; Angew. Chem. Int. Ed. 2018, 57, 15491-15495.
AG Biophotovoltaics Open Lecture
'Engineering cyanobacteria for direct solar chemical and fuel production'

Prof. Peter Lindblad
Microbial Chemistry
Uppsala University, Sweden
Cyanobacteria, prokaryotic microorganisms with basically the same oxygenic photosynthesis as higher plants, are excellent green cell microfactories for sustainable generation of renewable chemicals and fuels from solar energy and CO2. In the presentation I will visualize the concept green cell factories by discussing two examples: (i) Generation of functional semisynthetic [FeFe]-hydrogenases linking to the native metabolism in living cells of cyanobacteria cells, and (ii) Engineering cyanobacteria to produce the bulk chemical and blend-in molecule butanol from sunlight and CO2.
[FeFe] hydrogenases are the most efficient molecular catalysts known to date with regards to H2 production. Cyanobacteria harbor native [NiFe] hydrogenase(s) but no [FeFe] hydrogenase. A heterologously expressed [FeFe] hydrogenase was synthetically activated in a hydrogenase deficient strain of the cyanobacterium Synechocystis PCC 6803 (1). H2-evolution assays showed that the non-native, semi-synthetic enzyme links to the native metabolism in living cells. The artificial activation technology was expanded to a newly discovered [FeFe]-hydrogenase which when expressed in Synechocystis showed stable expression and significant H2 production under different environmental conditions (2). This semisynthetic [FeFe]-hydrogenase remained functional in vivo in cells of Synechocystis for several days. The developed technology opens up unique possibilities to investigate not only [FeFe]-hydrogenases but also other metalloenzymes in a photosynthetic microbial cell environment, completely bypassing the many challenges of e.g. biological maturation and regulations.
Cyanobacteria lack the butanol biosynthetic pathways and corresponding relevant gene(s). Introduction of a single gene encoding KivD results in isobutanol producing strains. Further engineering and long-term experiments demonstrated a maximal cumulative isobutanol titer of 1.2 gram per liter (3, unpublished). Similarly, introducing a complete 1-butanol pathway, together with additional enzymes to increase the pool of acetyl-CoA, resulted in cells with a cumulative titer of 4.8 g per L, and a maximal rate of 600 mg 1-butanol per L and day and carbon partitioning of 60% (4-6). Present progress towards a practical system will be presented and discussed.
(1) Wegelius et al 2018. Generation of a functional, semisynthetic [FeFe]-hydrogenase in a photosynthetic microorganism. Energy & Environmental Science 11: 3163-3167
(2) Wegelius et al 2021. Semisynthetic [FeFe]-hydrogenase with stable expression and H2 production capacity in a photosynthetic microbe. Cell Reports Physical Science 2: 100376
(3) Xie & Lindblad. 2022. Effects of expressing 2-keto acid pathway enzymes on photosynthetic isobutanol production. Microbial Cell Factories 21: 17
(4) Wichmann et al 2021. Engineering Biocatalytic Solar Fuel Production: The PHOTOFUEL Consortium. Trends in Biotechnology 39: 323-327
(5) Liu et al 2021. Engineering cyanobacteria for photosynthetic butanol production. In: Photosynthesis. Biotechnological Applications with Microalgae (Ed: Rögner) 2: 33-56. Walter de Gruyter GmbH, Berlin/Boston (6) Liu et al 2022. Current advances in engineering cyanobacteria and their applications for photosynthetic butanol production. Current Opinion in Biotechnology 73: 143-150
AG Biophotovoltaics Open Lecture
'Design meets evolution: Theory and practice for fine-tuned bioengineering'

Prof. Víctor de Lorenzo
Systems Biology Department
Centro Nacional de Biotecnología, CSIC
The prevailing view of biological evolution is not unlike bricolage/pastiche/tinkering—in sharp contrast with rational engineering. Yet, different paths often lead to solutions that coincide or converge whether they emerge from naturally-occurring evolution or rationally designed. Such a conjunction—often presented as a mere anecdote— in fact reveals the ability of biological systems to physically explore solution spaces and gravitate towards information-rich attractors, which can be found through different routes. This scenario evokes one of heterotic computing, a non-conventional type of data processing in which the solution to a problem is not delivered through numerical calculations but through its embodiment in a material object. Once left to undergo a physical process the object manages a large number of parameters for reaching a multi objective optimum. The course of information is thus a physical flow and the outcome is a physical currency. The consequences of this notion for bioengineering are remarkable, as it enables solutions to multi-objective optimization challenges not yet amenable to all-rational approaches. The ensuing technical question is how to bring about hyper-diversification not only of genomic sequences but also environmental and context-dependent parameters for securing the desired performance of a given synthetic device. This issue will be illustrated with a number of practical cases where naturally-occurring or artificially enhanced variability was key to find ideal outcomes to otherwise intractable design hitches of interest for industrial and environmental biotechnology. (1) Al-ramahi et al. (2021) ssDNA recombineering boosts in vivo evolution of nanobodies displayed on bacterial surfaces. Comms Biology 4: 1169. (2) Tas et al. (2020) Contextual dependencies expand the re-usability of genetic inverters. Nature Comms 12: 355.
(3) Espeso et al. (2020) An automated DIY framework for experimental evolution of Pseudomonas putida. Microb Biotechnol. 14: 2679-2685(4) Hueso-Gil et al. (2019) Multiple-site diversification of regulatory sequences enables inter-species operability of genetic devices. ACS Synth Bio 9: 104–114.
(5) Akkaya et al. (2019) Evolving metabolism of 2,4-dinitrotoluene triggers SOS-independent diversification of host cells. Env Microbiol 21: 314-326
(6) Pérez-Pantoja et al. (2013) Endogenous stress caused by faulty oxidation reactions fosters evolution of 2,4-dinitrotoluene-degrading bacteria. PLoS Genetics 9:e1003764
IP4 Science Talks
'Achieving sustainable wastewater treatment using a green technology'

Prof. Qilin Wang
School of Civil and Environmental Engineering
University of Technology Sydney, Australia
The FA technology increases the volatile solids destruction of anaerobic digestion by 12-26% and improves dewaterability of anaerobically digested sludge by 9-13%, which collectively cause a volume reduction of waste sludge by 15-21%. This reduces the cost of sludge disposal. The FA technology expands the treatment capacity of anaerobic digesters by 50%, which enables the WWTPs to accommodate population growth (i.e. increasing sludge production) and save the cost for the upgrade of WWTPs. In addition, the FA technology could enhance nitrogen removal from wastewater by 30%, facilitating treated wastewater to meet regulatory discharge standards.
