Workshop
Drug lifecycle control in Subsaharan Africa
From production to responsible safe disposal and elimination in wastewater treatment plants
(Med4Africa)

The aim of this workshop was a gathering of scientists, postdocs and doctoral students, as well as of stakeholders from academia, government authorities and industry, to initiate research activities on the drug lifecycle stages which are of relevance for the African continent.
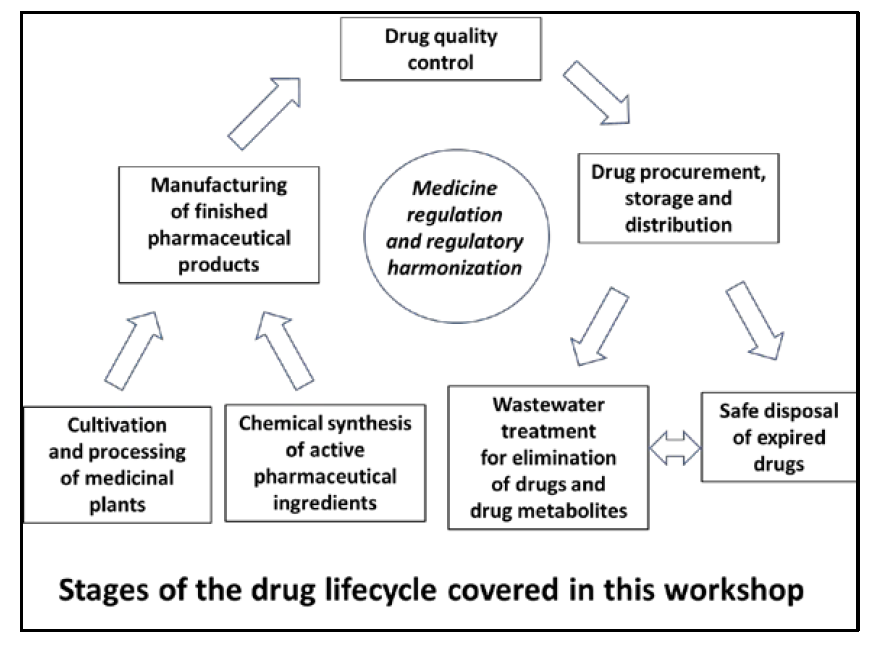
In the context of this workshop, drug lifecycle is understood as the stages from the production of active pharmaceutical ingredients and excipients, through drug manufacturing, quality control, drug procurement/storage/distribution, up to the safe disposal of expired drugs as well as wastewater treatment for removal of drugs and drug metabolites. Geographically, we concentrated on the United Republic of Tanzania, Ethiopia, Kenya, Botswana, Rwanda and Nigeria. All these countries are faced with major challenges in the topics named above, and, as a result, there is a great need for additional research, both in the interest of the availability, accessibility and affordability of medicines of good quality for therapeutic purposes, and in the interest of the environment and human, animal, and plant health. The additional aim of the workshop was to catalyze cooperative research proposals and activities by workshop participants across different countries and different fields of expertise.
BOOK OF ABSTRACTS
PRESENTATIONS

Welcome address
Production of Active Pharmaceutical Ingredients and Excipients
Nature-Derived Medicines
Drug Manufacturing
Drug Quality Control
Drug Procurement, Storage and Distribution (Logistics)
Safe Disposal of Expired Drugs
Wastewater Treatment to Remove Toxic and Environmentally Problematic Drug Substances and Metabolites
Medicine Regulation and Regulatory Harmonization
Case studies


ADDITIONAL USEFUL INFORMATION
Safety Guideline for Minilab Users
PRESS RELEASES

