Predicting the partition coefficients of neutral and ionic organic chemicals
Partition coefficients of neutral organic chemicals to structural proteins, plasma proteins, phospholipids and storage lipids as well as various solvents and technical sorbents can be estimated with the pp-LFER approach (Endo et al, 2013) based on molecular descriptors from our online data base (LSERD; http://www.ufz.de/lserd). This data base contains experimental descriptors for about 8000 chemicals. For all other chemicals a QSAR provides descriptor estimates which are, however, substantially less accurate than the experimental values.
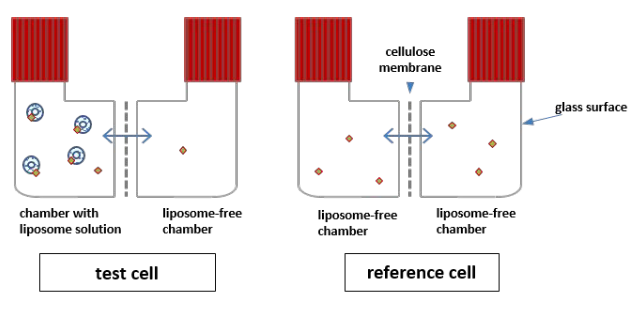 Measuring partition coefficients, from Ebert et al. 2020
Measuring partition coefficients, from Ebert et al. 2020
Not only neutral organic chemicals but also ionic species have a tendency to sorb to organic matrices like proteins and phospholipids. As for neutral chemicals, this partitioning behavior of ionic species must be quantitatively understood in order to assess their toxicokinetic profile. A mechanistic understanding of the sorption behavior of organic ions is much more complex though (Henneberger & Goss, 2019). So far, this field has only partly been covered by others and ourselves. We performed measurements for various environmentally relevant polyfluorinated chemicals in biological matrices (Allendorf et al. 2019, Ebert et al. 2020) and for organic amines in soil constituents (Droge and Goss 2013).
In cooperation with our department, the commercial software COSMOmic was extended for the application for ions (Bittermann et al. 2014). It is able to predict the partitioning between a phospholipid bilayer and water with an rmse = 0.7 log units for monovalent acids and bases (Bittermann et al., 2016).
Related own publications:
Allendorf, Flora, Urs Berger, Kai Uwe Goss, and Nadin Ulrich. 2019. “Partition Coefficients of Four Perfluoroalkyl Acid Alternatives between Bovine Serum Albumin (BSA) and Water in Comparison to Ten Classical Perfluoroalkyl Acids.” Environmental Science: Processes and Impacts 21 (11): 1852–63.
https://doi.org/10.1039/c9em00290a
Bittermann, Kai, Simon Spycher, Satoshi Endo, Larissa Pohler, Uwe Huniar, Kai Uwe Goss, and Andreas Klamt. 2014. “Prediction of Phospholipid-Water Partition Coefficients of Ionic Organic Chemicals Using the Mechanistic Model COSMOmic.” Journal of Physical Chemistry B 118 (51): 14833–42.
https://doi.org/10.1021/jp509348a
Bittermann, Kai, Simon Spycher, and Kai Uwe Goss. 2016. “Comparison of Different Models Predicting the Phospholipid-Membrane Water Partition Coefficients of Charged Compounds.” Chemosphere 144: 382–91.
https://doi.org/10.1016/j.chemosphere.2015.08.065
Droge, Steven T.J., and Kai Uwe Goss. 2013. “Ion-Exchange Affinity of Organic Cations to Natural Organic Matter: Influence of Amine Type and Nonionic Interactions at Two Different PHs.” Environmental Science and Technology 47 (2): 798–806.
https://doi.org/10.1021/es3033499
Ebert, Andrea, Flora Allendorf, Urs Berger, Kai Uwe Goss, and Nadin Ulrich. 2020. Membrane/Water Partitioning and Permeabilities of Perfluoroalkyl Acids and Four of Their Alternatives and the Effects on Toxicokinetic Behavior. Environmental Science & Technology. Vol. 54.
https://doi.org/10.1021/acs.est.0c00175
Henneberger, Luise, and Kai Uwe Goss. 2021. “Environmental Sorption Behavior of Ionic and Ionizable Organic Chemicals.” Reviews of Environmental Contamination and Toxicology 253: 43–64.
https://doi.org/10.1007/398_2019_37
