Investigating the role of active efflux in membrane permeability
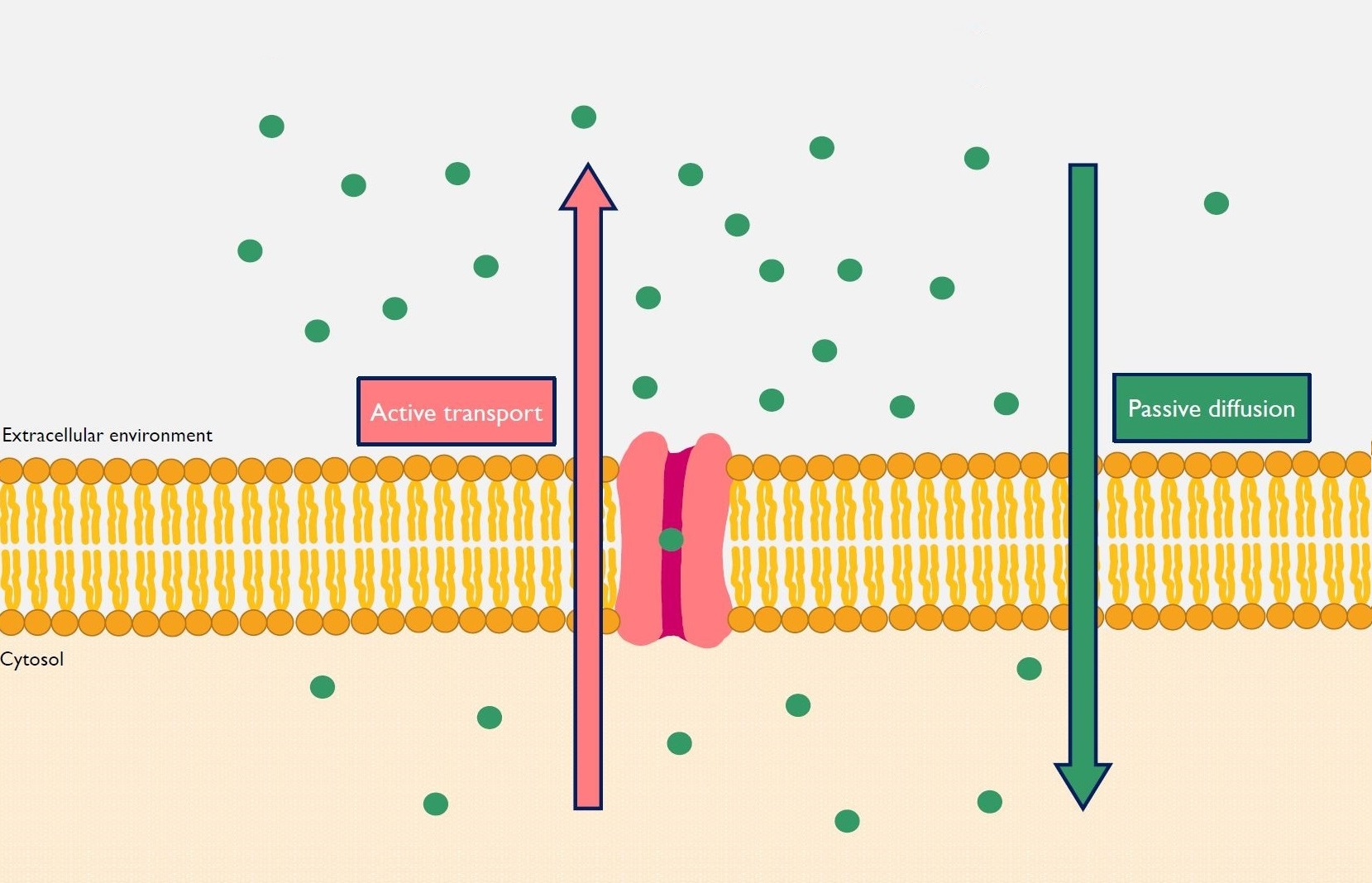
Our work on active transport branches of from our interest in membranes and our extensive investigations of passive permeability. In order to fully understand cellular membrane permeability, it is essential to also consider the active transport of compounds facilitated by transporter proteins embedded within the membrane. Apparent permeabilities that are obtained through the use of transwell experiments (or Caco-2/MDCK assays), are not only the in vitro gold-standard for assessing the uptake efficiency of drugs through the determination of intrinsic permeability values, but they are also used for determining whether compounds are substrates of certain efflux pumps.
 Role of active efflux in membrane permeability
Role of active efflux in membrane permeability
Building on our model for the prediction of the passive permeability of organic chemicals through Caco-2 and MDCK monolayers (Bittermann and Goss 2017), which was refined to account for concentration-shift effects (Dahley et al. 2023, see Passive Membrane Permeability project), we extended the model to include active transport facilitated by an efflux pump embedded within the apical membrane. As of yet, transwell assay data is primarily used to simply qualitatively state whether a compound is a substrate of a given efflux transporter. However, we hope that this model will ultimately enable the determination of an intrinsic value for active transport (analogous to an intrinsic permeability value for passive transport) that can be used to accurately quantify active efflux.
As part of our first aim, we investigated the efflux ratio (ER), which is the experimental metric determined from bidirectional transwell assays. The ER is simply the measure of apparent permeability in the basolateral-to-apical direction, relative to that in the apical-to basolateral direction. When the ER is greater than a pre-defined threshold (commonly 2), then it is qualitatively stated that efflux occurs, and that the compound is thus a substrate of the transporter under investigation. Other studies have attempted to enable the utilisation of this metric quantitatively by deriving a relationship that expresses the ER as a function of the intrinsic membrane permeability of the membrane as well as the permeability of carrier-mediated efflux. However, previous derivations of this relationship failed to consider the influence of additional transport resistances such as the aqueous boundary layers (ABLs) and the filter on which the monolayer is grown. As such, the first aim of our project was to include these factors into the model and to evaluate both mathematically and experimentally, any potential impact on the ER. Ultimately, we showed that the previously-established ER relationship does indeed hold for a more realistic scenario that does not neglect the ABLs/filter. Furthermore, we also mathematically showed how paracellular transport affects the ER, and we experimentally confirmed that paracellular dominance reduces the ER to unity and can mask potential efflux.
Our second aim, where our focus now lies, is to determine whether the ionic or neutral species of a compound is favoured by the efflux transporter. Knowing which fraction of a chemical the transporter predominantly acts on is essential for correctly modelling its action. Once again, our approach is two-pronged: we perform transwell experiments across a pH range to see how this affects the ER, and we compare this with model simulations based on different assumptions of which species fraction the transporter preferentially acts on.
Related own publications:
Kotze, Soné, Andrea Ebert, and Kai Uwe Goss. 2024. “Effects of Aqueous Boundary Layers and Paracellular Transport on the Efflux Ratio as a Measure of Active Transport Across Cell Layers.” Pharmaceutics 16 (1). https://doi.org/10.3390/pharmaceutics16010132
Kotze, Soné, Kai-Uwe Goss, and Andrea Ebert. 2024. “The PH-Dependence of Efflux Ratios Determined with Bidirectional Transport Assays across Cellular Monolayers.” International Journal of Pharmaceutics: X 8: 100269.
https://doi.org/10.1016/j.ijpx.2024.100269
