Electrode less water „electrolyses“ using a radio frequency (RF) plasma
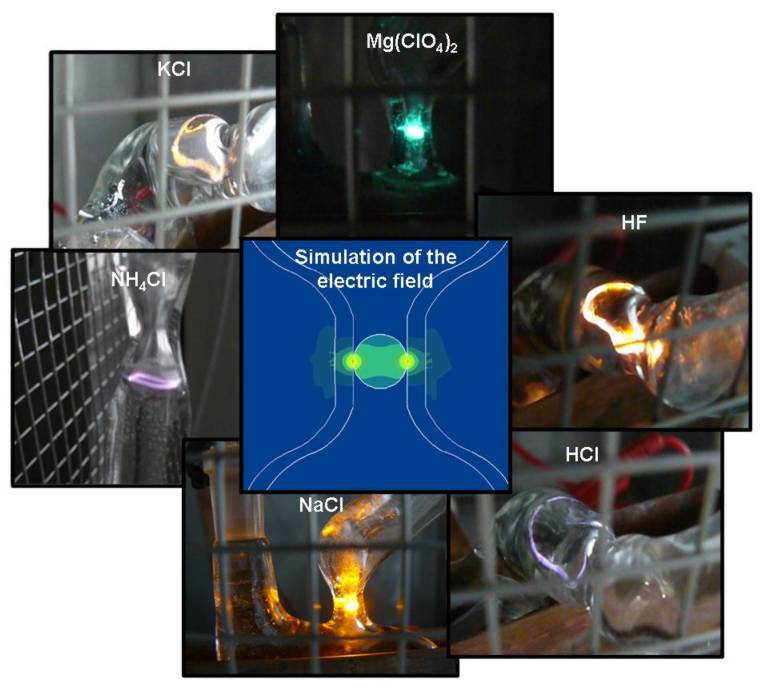
Electrode less water “electrolyses” or more precisely water dissociation using a radio frequency plasma. This phenomenon is also called “burning water”.
Due to a strongly focused electro magnetic field a plasma can be initiated dissociating water molecules into hydrogen and oxygen. Depending on the electrolyte used the water “burns” in different colours.
Beside applied research in the Department of Environmental Engineering also new and fundamental effects are investigated in order to evaluate their application potential. A good example for such an effect is the electrode less water “electrolysis” in a radio frequency (13.56 MHz) plasma, also called “burning water”. In contrast to well known common electrolysis water is here dissociated into hydrogen and oxygen without direct contact of water with the RF electrodes, thus, avoiding corrosion of and undesired chemical reactions at the electrode surfaces.
Water dissociation is caused by on a
non thermal plasma
initiated by strongly focused electromagnetic fields at the gas-liquid interface of a water vapour bubble. A characteristic feature is the application of electrolyte solutions in a concentration range of some weight percent. Therefore, also ordinary sea water can be used without any pre-treatment. Depending on the electrolyte used the water ‘burns’ in different colours representing emission wavelengths that are characteristic for the dissolved salt.
Beside mechanistic studies potentials and constraints of an application of the electrode less water “electrolysis” are being investigated. In principle, the effect can be used for hydrogen production, but energy conversion efficiencies are currently not competitive yet. A second field of application could the removal of organic pollutants in water, since highly reactive oxidative and reductive active species (e.g. radicals) are produced during the plasma process.
