Transfer-News 2026
Environmental chemicals on social media: @ufz_safe explains research in everyday terms
With its Instagram channel @ufz_safe, UFZ shares reliable information on environmental chemicals where many people look for guidance day to day: on social media. Run by Nicole Meyer and Florence Fischer (Department of Environmental Immunology), the channel addresses audiences of all ages.
Posts explore questions around environmental chemicals in food, air, water and soil, helping to put current research findings into context and offering practical guidance for more informed everyday choices. The aim is to strengthen science communication, address uncertainties and counter misinformation – clearly, accessibly and without jargon.
January
2025
Licence agreement with MAXX GmbH: sales and distribution of the automatic UFZ water sampler to start in 2026
 As part of a long-standing collaboration with MAXX Mess- und Probenahmetechnik GmbH, a cost-effective automatic sampler for isotope-hydrological applications was developed within a transfun® project and successfully tested at UFZ in Halle. The modular autosampler can be adapted to different deployment conditions and offers a practical solution for environmental monitoring, particularly for public authorities, universities and other research institutions. Key features include evaporation-free sample storage until analysis, autonomous battery-powered operation, sampling of precipitation as well as surface water and groundwater, low maintenance requirements, and straightforward programming in the field.
As part of a long-standing collaboration with MAXX Mess- und Probenahmetechnik GmbH, a cost-effective automatic sampler for isotope-hydrological applications was developed within a transfun® project and successfully tested at UFZ in Halle. The modular autosampler can be adapted to different deployment conditions and offers a practical solution for environmental monitoring, particularly for public authorities, universities and other research institutions. Key features include evaporation-free sample storage until analysis, autonomous battery-powered operation, sampling of precipitation as well as surface water and groundwater, low maintenance requirements, and straightforward programming in the field.
Further information: MAXX GmbH
August
20 years of Isodetect – long-standing partnership with UFZ in environmental analytics
 Isodetect GmbH was founded in 2005
as a joint spin-off from UFZ Leipzig and the Helmholtz Centre Munich, supported
by the Helmholtz spin-off fund. After early activities in Munich and Leipzig,
the company established its base in BioCity Leipzig in 2014, combining state-of-the-art
isotope analytics with microbiological methods and building a strong profile in
site investigation for remediation.
Isodetect GmbH was founded in 2005
as a joint spin-off from UFZ Leipzig and the Helmholtz Centre Munich, supported
by the Helmholtz spin-off fund. After early activities in Munich and Leipzig,
the company established its base in BioCity Leipzig in 2014, combining state-of-the-art
isotope analytics with microbiological methods and building a strong profile in
site investigation for remediation.
A key focus of the collaboration is the investigation of contaminated sites and drinking-water aquifers, closely linked with UFZ’s Laboratories for Stable Isotopes (LSI). Early on, the groundwork was laid for close cooperation and ongoing exchange through joint activities and regular professional dialogue. In recent years, this has been further strengthened through six publicly funded joint research projects and 15 research assignments. Today, Isodetect experts also contribute as mentors to the UFZ support programme transfun®.
On the occasion of Isodetect’s 20th anniversary, UFZ welcomes the continuing partnership and extends its best wishes for the future.
July
Innovative UFZ technology removes PFAS from water sustainably
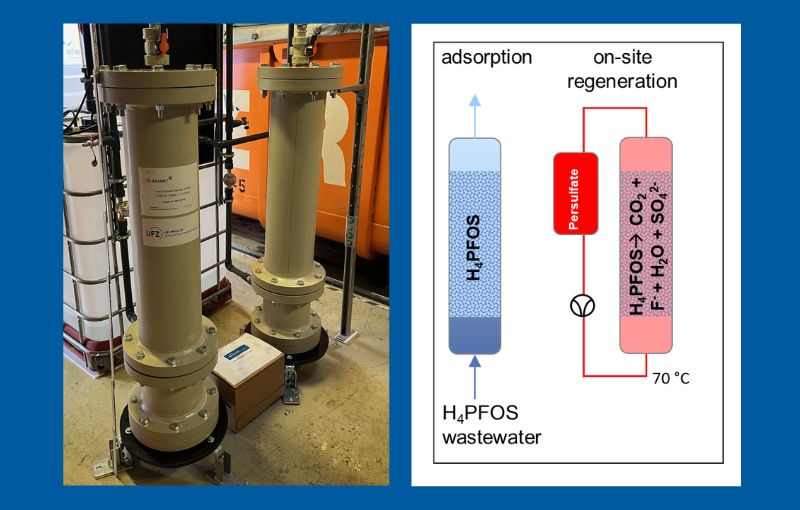 With the granting of the Australian patent, the last property right within the patent family EP3873659 ‘Process for the removal of polyfluorinated organic compounds from water using an adsorbent and its regeneration’ has now been granted. This means that the innovative process is patented in a total of 23 countries.
With the granting of the Australian patent, the last property right within the patent family EP3873659 ‘Process for the removal of polyfluorinated organic compounds from water using an adsorbent and its regeneration’ has now been granted. This means that the innovative process is patented in a total of 23 countries.
The protected technology enables the efficient removal of PFAS from water – particularly from industrial wastewater and contaminated groundwater. Zeolites are used as adsorbents, which can be regenerated on site by wet chemical oxidation after adsorbing the pollutants.
The process is characterised by several technical advantages:
• High adsorption capacity: zeolites have a particularly good adsorption capacity for PFAS, even in case of complex water contamination.
• Sustainable regeneration: the oxidation with persulphate completely mineralises the pollutants, thus reducing the disposal effort of contaminated materials.
• Practical application: The technology is mobile and does not require expensive high-temperature equipment, which saves transport costs and energy.
• Material longevity: A controlled pH regime during regeneration ensures stable adsorption performance over several cycles of use.
The process is currently being tested by industrial partner Diepersdorf Plastic Manufacturing GmbH at its production site. The implementation is being carried out in the ZeoPFAS project and is funded by the UFZ innovation fund transfun®.
More information about ZeoPFAS: https://www.ufz.de/index.php?de=51538
June 2025
BODIUM4Farmers: Web Application Combines Yield Security and Soil Protection in Agriculture
 © https://www.bonares.de/
As part of the BonaRes project, the web application BODIUM4Farmers was developed to support agricultural businesses in the sustainable management of their land. The application is based on the systemic soil model BODIUM, which was designed to depict the complex interactions between soil, plants, and management practices.
© https://www.bonares.de/
As part of the BonaRes project, the web application BODIUM4Farmers was developed to support agricultural businesses in the sustainable management of their land. The application is based on the systemic soil model BODIUM, which was designed to depict the complex interactions between soil, plants, and management practices.
With BODIUM4Farmers, farmers can simulate the long-term effects of different management scenarios on key soil functions such as yield capacity, water storage, nutrient budget, and carbon sequestration. This involves considering site-specific soil data, weather information, and historical management data to enable realistic and practical forecasts. The goal is to strike a balance between economic requirements and soil resource protection – particularly in the face of climate change and changing legal frameworks.
The application was developed in close collaboration with agricultural practitioners and will be available free of charge from summer 2025. In follow-up projects, BODIUM4Farmers will be further developed in collaboration with businesses to create tailored, sustainable management concepts. Thus, the project significantly contributes to promoting a future-proof and resource-efficient agriculture.
May 2025
Licence agreement concluded: Isocitric acid on the verge of industrial application
 © Adobe Stock#107081488
In a multi-year collaboration, Andreas Aurich and Steffi Hunger, from the UFZ Department of Systemic Environmental Biotechnology (SUBT), and ChiroBlock GmbH have developed and optimised an innovative, yeast-based bioprocess for the sustainable production of isocitric acid (ICA) from ethanol or cooking oil and a product separation. The bioprocess is characterised by its robustness, long-term stability over several weeks, high yields and high selectivity for the target product.
© Adobe Stock#107081488
In a multi-year collaboration, Andreas Aurich and Steffi Hunger, from the UFZ Department of Systemic Environmental Biotechnology (SUBT), and ChiroBlock GmbH have developed and optimised an innovative, yeast-based bioprocess for the sustainable production of isocitric acid (ICA) from ethanol or cooking oil and a product separation. The bioprocess is characterised by its robustness, long-term stability over several weeks, high yields and high selectivity for the target product.
While it has not been possible to synthesise chiral ICA efficiently as a natural substance, the new biotechnological approach opens up completely new possibilities. Compared to its sister product, citric acid, which is already well established in industry, isocitric acid was previously difficult to produce and a high-priced product. With the new process, economically relevant quantities can now be produced, opening up new areas of application.
An important milestone has now been reached with the conclusion of a licence agreement between ChiroBlock and the UFZ. This will enable ChiroBlock to further scale up the process in order to produce isocitric acid in larger quantities on an industrial scale and to drive forward certification in preparation for market launch.
If you are interested in the product, please contact ChiroBlock.
April 2025
More precise pollen data for better forecasts: UFZ and iDiv integrate measurement data into European database
 UFZ/iDiv pollen trap in Leipzig © EAN, Konstantin Albrecht
It is pollen season again, and many people are suffering from allergies.
Accurate and spatially well resolved pollen data is crucial to provide early
warnings to those affected and minimise health risks. It improves forecasts,
medical care and individual protective measures.
UFZ/iDiv pollen trap in Leipzig © EAN, Konstantin Albrecht
It is pollen season again, and many people are suffering from allergies.
Accurate and spatially well resolved pollen data is crucial to provide early
warnings to those affected and minimise health risks. It improves forecasts,
medical care and individual protective measures.
Our expert, Dr Susanne Dunker, conducts research at the Helmholtz Centre for Environmental Research (UFZ) in the Department of Physiological Diversity and at the German Centre for Integrative Biodiversity Research (iDiv). She has developed an innovative, automated method for pollen analysis that combines image-based flow cytometry with deep learning to efficiently identify and quantify pollen. However, until the method is recognised as standard, the pollen data will still be determined using the classic method of microscopic counting, as will the pollen data that has now been integrated into the EAN database.
There are hardly any pollen traps in Central Germany. Susanne Dunker and her team started a collaboration with Leipzig University Hospital as early as 2019 and together with Dr. Jan Bumberger (Department Monitoring and Exploration Technologies), the team established a monitoring station on the hospital's roof. The pollen data should provide new insights into the relationship between air quality, pollen diversity and allergenicity in urban areas.
The next step is for the UFZ and iDiv to integrate the pollen data collected since 2019 into the European Aeroallergen Network (EAN) database. The EAN combines data from over 600 pollen monitoring stations in Europe and makes it available to a broad scientific community and interested stakeholders, such as the Earth Information data provider Copernicus.
And what are the plans for the future? Susanne Dunker and her team are working on continuously improving the automated process for pollen analysis in order to minimise error rates. The potential is huge, as automated analysis allows high-throughput measurements and saves a lot of time compared to microscopy-based counting. In addition, the data from two other Leipzig pollen trap sites - at the Leipzig Auwaldkran and the one in Leipzig-Mitte, which is a reference station of the State Office for Environment, Agriculture and Geology (LfULG) - could be made available in EAN in the future.
Pollen data is of interest to a wide range of sectors: not only for allergy sufferers and doctors, but also for meteorological services, which provide pollen forecasts, for pharmaceutical companies, to develop targeted medications and allergy immunotherapies, for agriculture and forestry, to assess plant flowering and pollination processes, and for cities and municipalities, to adapt their green space planning and reduce allergenic plants.
Further information:
Link to
the EAN database: https://ean.polleninfo.eu/Ean/
Publication: Monitoring and perception of allergenic pollen in urban park environments https://www.sciencedirect.com/science/article/pii/S0169204624001324
March 2025
New Approaches to Reduce Bird Tests in Chemical Assessments
 Cranes © Manfred Ziegler
Two teams led by Dr Stephan Schaller (ESQlabs GmbH) and Dr Jo Nyffeler (UFZ Chemicals in the Environment) have received £300,000 in funding to develop new approaches for evaluating the potential risks of chemicals to birds as part of the Wings of Change CRACK IT Challenge 2024. The challenge aims to reduce the need for live birds in research, as a large number of birds are currently used in toxicity studies to investigate acute and chronic effects of chemicals on bird species.
Cranes © Manfred Ziegler
Two teams led by Dr Stephan Schaller (ESQlabs GmbH) and Dr Jo Nyffeler (UFZ Chemicals in the Environment) have received £300,000 in funding to develop new approaches for evaluating the potential risks of chemicals to birds as part of the Wings of Change CRACK IT Challenge 2024. The challenge aims to reduce the need for live birds in research, as a large number of birds are currently used in toxicity studies to investigate acute and chronic effects of chemicals on bird species.
In the two-stage challenge, the teams will initially spend nine months analysing existing avian toxicity data to develop new methodologies. In the subsequent second phase, with up to £1.5 million in additional funding, the projects will be further developed over three years and integrated into a framework that reduces the number of animals used for risk assessments.
The Wings of Change Challenge is supported by six international sponsors, including BASF, Bayer Crop Science, Corteva, and Syngenta. In addition to financial support, the challenge partners, the American Chemistry Council and the Health and Environmental Sciences Institute, provide additional resources to promote method development. The CRACK IT Challenge is a funding programme by the NC3Rs that fosters collaboration between industry and science to reduce or replace animal testing.
By integrating new approaches into regulatory procedures, environmental and animal welfare standards could be strengthened in the long term. The close collaboration between teams, sponsors, and partners not only enables the reduction of animal testing but also the development of practical solutions that can make future risk assessments more efficient and ethically responsible.
January 2025