MADDOMS
Novel ultra-high resolution Mass spectrometric Approaches to Decipher structural and physico-chemical Drivers of soil Organic Matter Stabilization

Priority programme 2322 ‘‘SoilSystems: Systems ecology of soils – energy discharge modulated by microbiome and boundary conditions” funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation). Link
Members from the Department of Analytical Chemistry at UFZ: Dr. Oliver Lechtenfeld (PI), Dr. Carsten Simon (Postdoc), Konstantin Stumpf (PhD candidate), Prof. Dr. Thorsten Reemtsma (Head of Dept.)
External members: Dr. Marion Schrumpf (Max Planck Institute for Biogeochemistry, Jena, Germany), Prof. Dr. Robert Mikutta (Soil Science and Conservation, Martin Luther University Halle-Wittenberg, Germany), Dr. Klaus Kaiser (Soil Science and Conservation, Martin Luther University Halle-Wittenberg, Germany)
Overview. MADDOMS is a project within the priority programme 2322 ‘‘SoilSystems: Systems ecology of soils – energy discharge modulated by microbiome and boundary conditions” funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation). In this SPP, soil is seen as a highly complex, open thermodynamic system in which soil ecosystem structure, function and stability are dependent on energy discharge and consumption. Organic matter and microbial biomass in soils can be viewed as dissipative structures in this framework. The three main hypotheses of the SPP are:
1) The soil microbiome is a key modulator of energy dissipation and matter turnover (contributing to the formation of SOM via metabolism, recycling and necromass accumulation) and this follows specific energy use channels.
2) Inputs of energy and matter, their discharge and consumption shape the biological complexity of soils, for example microbiome diversity or foodweb organization.
3) Boundary conditions (e.g., oxygen or nutrient availability) and mineral composition shape the channels that are available for energy and matter use.
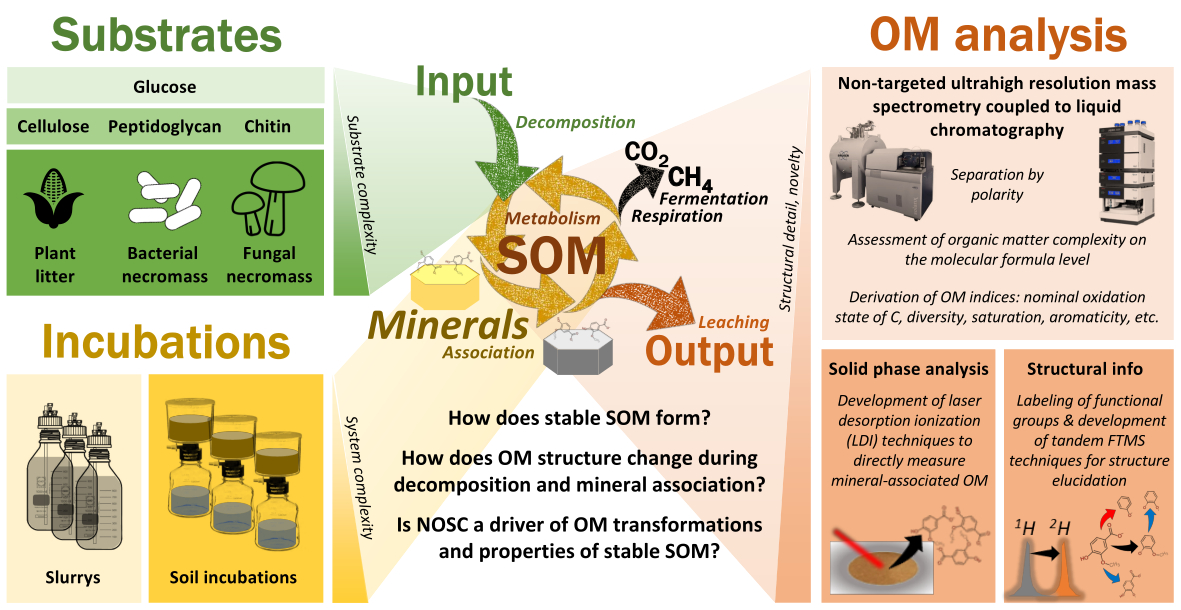
Rationale. MADDOMS combines incubation setups with development of novel analytical tools to assess SOM formation and composition changes (Figure 1). The project aims to establish new applications of molecular-level information by ultrahigh resolution mass spectrometry in soil science: structural properties (via tandem MS and isotope labeling approaches) and surface-associated molecules (via laser desorption techniques).
Nominal oxidation state as a proxy of SOM stability. Liquid-chromatography coupled to ultrahigh resolution mass spectrometry allows to access metabolic imprints of decomposer activities in organic matter [1]. These imprints include properties such as molecular weight, saturation, oxidation, polarity, aromaticity, and energy content (via nominal oxidation state of carbon, NOSC). Especially NOSC distributions of SOM are not well constrained which is why a central question of SOM persistence is not solved – why does SOM formation produce favorable substrates that are apparently not consumed but can persist [2]? MADDOMS will contribute to a better understanding of the thermodynamic basis of SOM formation through complementary techniques that assess energy and matter turnover in soils (Hypothesis 1).
Organic matter as a mirror of soil functional complexity. Soils are open, multiphase, heterogenous and living systems that experience frequent changes in boundary conditions [3, 4]. These systems are characterized by parallel and interconnected processes that produce highly complex assemblages of organic molecules [5,6]. MADDOMS will contribute to an improved understanding of SOM’s functional complexity and how it responds to substrate type, decomposition, redox state and exposition to mineral surfaces. Functional complexity will here be viewed not only on the molecular formula level [7], but also on the structural level [8–10]. Another layer of understanding functional complexity will be provided by comparing dissolved vs. mineral-associated molecular assemblages (Hypothesis 2) [11,12].
Boundary conditions control the formation pathways and composition of stable SOM. Soil organic matter stability or persistence is now viewed as an ecosystem property rather than a sole matter of differential recalcitrance of specific structures or biomaterials [13–15]. Within the SPP, incubation setups will provide a possibility to test both effects on the basis of three major substrate categories (plant, bacteria, fungi). MADDOMS will access the effects of substrate decomposition on molecular and structural properties of the produced organic matter and compare it with signatures of native SOM in the four SPP sites. Long-term incubations will provide insights into external (boundary condition) and internal (biochemical potential of the decomposer community) constraints on these processes and their outcome in terms of molecular and structural features (Hypothesis 3).
Further reading
[1] Han, L.; Kaesler, J.; Peng, C.; Reemtsma, T.; Lechtenfeld, O. J. (2021): Anal. Chem. 93: 1740. Link
[2] Gunina, A.; Kuzyakov, Y. (2022): Glob. Chang. Biol. 28: 2169. Link
[3] Addiscott, T. (2010): Geoderma. 160: 31. Link
[4] Sierra, C. A.; Müller, M.; Metzler, H.; Manzoni, S.; Trumbore, S. E. (2017): Glob. Chang. Biol. 23: 1763. Link
[5] Lehmann, J.; Hansel, C. M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J. P.; Torn, M. S.; Wieder, W. R.; Kögel-Knabner, I. (2020): Nat. Geosci. 13: 529. Link
[6] Lehmann, J.; Kleber, M. (2015): Nature 528: 60. Link
[7] Mentges, A.; Feenders, C.; Seibt, M.; Blasius, B.; Dittmar, T. (2017): Front. Mar. Sci. 4: 194. Link
[8] Kostyukevich, Y.; Kononikhin, A.; Zherebker, A.; Popov, I.; Perminova, I.; Nikolaev, E. (2014): Anal. Bioanal. Chem. 406: 6655. Link
[9] Zherebker, A.; Kostyukevich, Y.; Kononikhin, A.; Kharybin, O.; Konstantinov, A. I.; Zaitsev, K. V.; Nikolaev, E.; Perminova, I. (2017): Anal. Bioanal. Chem. 409: 2477. Link
[10] Simon, C.; Dührkop, K.; Petras, D.; Roth, V.-N.; Böcker, S.; Dorrestein, P. C.; Gleixner, G. (2022): Environ. Sci. Technol. 56: 11027. Link
[11] Lohse, M.; Haag, R.; Lippold, E.; Vetterlein, D.; Reemtsma, T.; Lechtenfeld, O. J. (2021): Front. Plant Sci. 12: 753812. Link
[12] Giannopoulos, K.; Benettoni, P.; Holbrook, T. R.; Reemtsma, T.; Wagner, S.; Lechtenfeld, O. J. (2021): Environ. Sci. Nano 8: 2336. Link
[13] Marschner, B.; Brodowski, S.; Dreves, A.; Gleixner, G.; Gude, A.; Grootes, P. M.; Hamer, U.; Heim, A.; Jandl, G.; Ji, R.; Kaiser, K.; Kalbitz, K.; Kramer, C.; Leinweber, P.; Rethemeyer, J.; Schäffer, A.; Schmidt, M. W. I.; Schwark, L.; Wiesenberg, G. L. B. (2008): J. Plant Nutr. Soil Sci. 171: 91. Link
[14] Schmidt, M. W. I.; Torn, M. S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I. A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D. A. C.; Nannipieri, P.; Rasse, D. P.; Weiner, S.; Trumbore, S. E. (2011): Nature 478: 49. Link
[15] Roth, V.-N.; Lange, M.; Simon, C.; Hertkorn, N.; Bucher, S.; Goodall, T.; Griffiths, R. I.; Mellado-Vázquez, P. G.; Mommer, L.; Oram, N. J.; Weigelt, A.; Dittmar, T.; Gleixner, G. (2019): Nat. Geosci. 12: 755. Link
