OSIRIS ITS Stakeholder Workshop
Optimised strategies for the risk assessment of chemicals
According to REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals), the new European legislation on chemicals and their safe use, all industrial chemicals produced or imported in quantities above 1 t/y have to be evaluated regarding their toxicological and ecotoxicological effects. Considering the currently used testing schemes, this procedure is expected to result in a significant increase in animal tests. However, REACH also aims at reducing animal testing where possible.
OSIRIS is developing Integrated Testing Strategies (ITS) considering both non-test and test information and thus combining different approaches for the hazard and risk evaluation of chemicals. ITS shift risk assessment from a “box-ticking” approach with extensive animal testing to a more effi cient, context-specifi c and substance-tailored approach. The underlying principle is to take advantage of existing information, to group information about similar substances, and to integrate exposure considerations in the decision making.
The complementary alternative approaches considered include:
• chemical and biological read-across
• qualitative/quantitative structure-activity relationships (QSAR)
• in vitro testing
• (existing) in vivo information
• chemoassays
• omics
• thresholds of toxicological concern (TTC)
• exposure analysis and exposure-based waiving.
The different information is weighted and the respective uncertainties taken into account in a Weight of Evidence approach.
OSIRIS Webtool
The methods and ITS developed are implemented in the webbased OSIRIS Tool, which will be made available to the public for end-users from industry, regulatory authorities and academia.
The following endpoints are included in the Webtool:
• skin sensitisation
• mutagenicity & carcinogenicity
• repeated dose toxicity
• aquatic toxicity
• bioconcentration factor.
Two uncertainty reasoning schemes are implemented with the OSIRIS Consensus Tool: Bayesian Networks and Dempster-Shafer theory of evidence. A Chemical Space Navigation Tool has been integrated as visual aid for pre-screening tasks.
The Webtool also includes interfaces to locally installed QSAR software for generating in silico predictions including information about respective application domains (ChemProp, OECD (Q)SAR Application Toolbox, ... ).
The OSIRIS Webtool indicates what tests (if any) should be performed in order to satisfy REACH data requirements. Data used and decisions taken are documented.
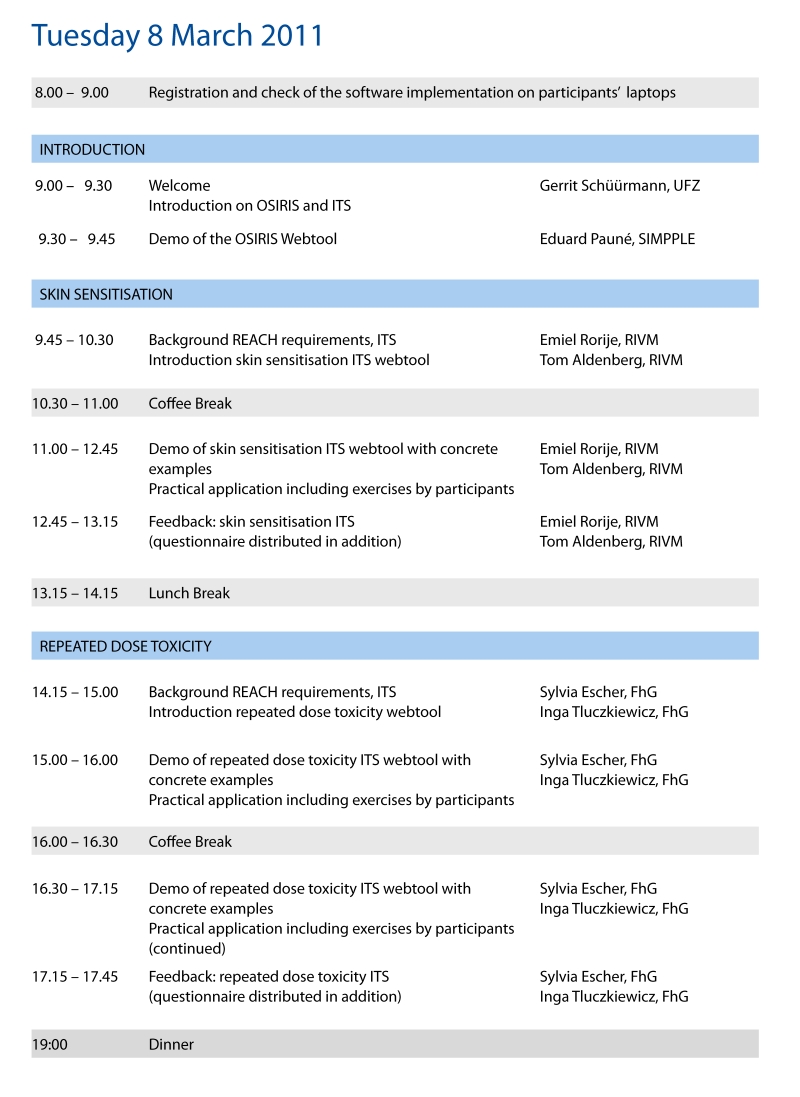
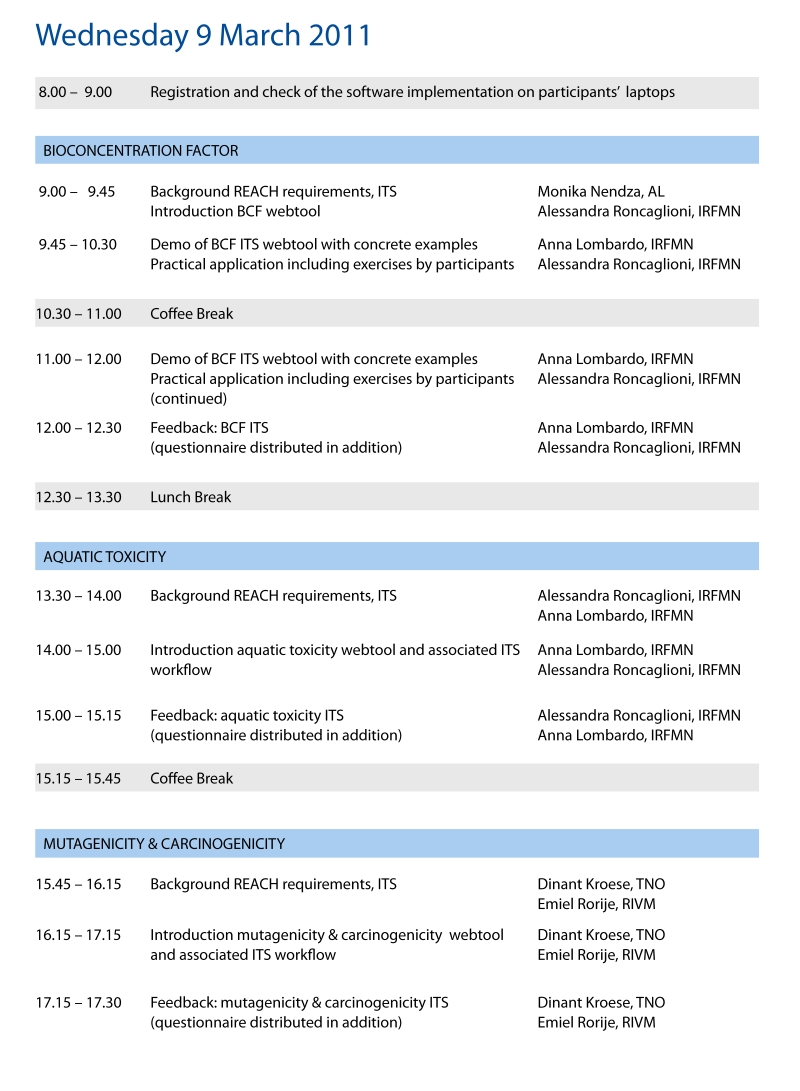
Programme