Press release December 14th, 2005
Using REACH as an opportunity to find alternatives to animal experiments
A need for intelligent test strategies instead of blindly testing everything
Leipzig. Scientists are demanding an intelligent strategy for the testing of numerous chemicals that will need to be conducted over the next twelve years as part of the implementation of REACH, the EU chemicals regulation. Only by employing alternative tests and theoretical testing methods, and by linking different approaches and information can the aim of reducing animal experiments be achieved despite the growing number of tests required.

The zebrafish. UFZ research groups are working on intelligent test strategies with fish embryos as an alternative to
standard test programmes for chemicals on adult fish.
Foto: André Künzelmann, UFZ
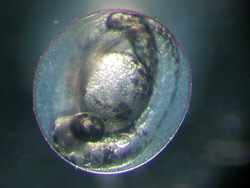
In a test tube, researchers can, for example, investigate how chemicals affect the embryos in fish eggs.
Foto: André Künzelmann, UFZ

Scientists use the Daphnia test for the risk assessment of chemicals on water organisms.
Foto: André Künzelmann, UFZ

Everyday we get in contact with chemicals, for example the contact of our skin with textiles.
Foto: André Künzelmann, UFZ

REACH stands for Registration, Evaluation and Authorisation of Chemicals. In future REACH should ensure that the whole life of a
chemical from producer to consumer can be understood and made safe. Registration is controlled by a new EU Chemicals Agency in Helsinki.
Foto: André Künzelmann, UFZ
"If the whole lot – estimated to be about 20 000 substances – is just put through the standard test programme, there is a danger that particularly risky substances will not be tested until ten years’ time", warns Prof. Gerrit Schüürmann of the Centre for Environmental Research Leipzig-Halle (UFZ), adding that it is also a question of making optimum use of existing test resources. For instance, with new models – so-called QSARs – it is possible to filter out potentially hazardous substances. These can then be investigated more thoroughly using alternative test methods. This also applies to substances that are subject to authorisation and produced in small quantities and which therefore do not come under the standard test programme. In general, it is necessary to bring together existing information in order to limit the test volume to what is actually necessary and to avoid unnecessary duplicate testing. A requirement for the industry to publish non-competition-sensitive data could also help avoid animal experiments, according to the UFZ scientists. At the moment, experts estimate that the implementation of REACH will involve an extra 1.5 million animal experiments.
The aim of the research at the UFZ is to replace the standard test programme for chemicals with an intelligent test strategy. For this it is necessary to link information about the toxicity of substances with other information and the frequency of their occurrence in the environment in order to develop a tailor-made test programme for each substance. "In this way we intend to achieve at least the same level of safety at a lower cost and with fewer animal experiments", says Prof. Gerrit Schüürmann. However, this can be achieved only by combining various components. These include for instance, focusing the test programme on the areas in which the substance in question is found, the systematic inclusion of information about similar substances, and making greater use of methods for the early analysis of possible mechanisms of action. Promising approaches for getting a grip on the large number of tests necessary for REACH economically and with as few additional animal experiments as possible include both experimental in vitro tests and computer-based models (QSARs).
‘In vitro’ as a replacement for some animal experiments
Until now, fish have accounted for a relatively small proportion of total animal experiments. Yet the number of fish tests will rise significantly over the next few years since they are a prerequisite for the authorisation of chemicals under the legislations for chemicals and plant protection. For this reason, various UFZ research groups are working on alternatives to experiments with adult fish. ‘In vitro’ comes from the Latin and means ‘in glass’. In a test tube, researchers can, for example, investigate how chemicals affect the embryos in fish eggs. The test with the embryos of the zebrafish Danio rerio is now approved, at least in Germany, as an alternative to the fish test for monitoring industrial effluents. Approval was achieved, in part, as a result of research carried out by the scientists working with Dr. Rolf Altenburger at the UFZ. "At the UFZ we are now investigating whether this test can be extended", says Dr. Kristin Schirmer. "In the BMBF GenDarT project we are using cutting-edge molecular biological methods to investigate whether gene activity in the embryo allows predictions to be made concerning chronic toxicity in the fish".
Another approach is the analysis of fish embryo proteins (proteomics) in order to identify so-called biomarkers as indicators or warning signals for
probable effects. Such kinds of protein analyses are currently being established by Dr. Eberhard Küster in close collaboration with another UFZ working
group as part of the EU PROTECTOR project.
Fish cell lines, that is permanently growing cells obtained from fish tissues, are another alternative. "Together with Canadian scientists, we have been
able to demonstrate that there is a correlation between toxic effects on a cell line from the gills and the whole fish", says Kristin Schirmer. Using cell
cultures like these, which can be representative of various fish organs, it is possible to conduct a series of tests without having to kill a fish every time.
"One shortcoming from our point of view is that for chemicals with an annual production of between 1 and 10 tonnes, the only risk assessment for water organisms provided for by REACH is the Daphnia test. Fish tests, for instance, are only required for substances with higher annual production", explains Dr. Eberhard Küster. Yet particularly powerful substances are usually produced in small quantities and are therefore excluded from the evaluation. "Even a single substance can have a very powerful effect on an ecosystem." Triclosan, a commonly used bactericide, was found in laboratory experiments to produce a dramatic shift in algae populations, which has so far not been described. Since algae are at the beginning of the food chain in the water, this shift in turn can have impacts on all living creatures that come after them in the food chain. Standardised algae tests in accordance with the DIN standard take between 72 hours and several days, depending on the species. The UFZ scientists are therefore working on methods that will reduce the length of the tests or dramatically increase the number of samples that can be assessed at the same time ("high-throughput bioassays"). These studies look both at microscopically small algae and large species of algae like the stonewort, and also at higher plants like duckweed. This means that all the important plant levels of an aquatic ecosystem are included. With these analyses it is possible to test up to 96 samples much faster than with conventional tests. The ecotoxicologists hope that this test will one day replace the standard test for higher aquatic plants.
Tailor-made test programmes using QSARs
QSARs (Quantitative or Qualitative Structure-Activity Relationships) are models that predict the potency (quantitative) or mechanism of action (qualitative) of a substance from its chemical structure. This makes it possible, for instance, to make deductions about toxic properties and therefore to prevent animal experiments. The European Commission’s Joint Research Centre (JRC) therefore intends to increase activities to establish QSARs over the coming years. After all, the JRC estimates that QSAR models alone can save the industry between 740 million and 940 million euros out of the estimated maximum of 2.4 billion or so euros that REACH would entail in test costs. QSARs should make it easier to carry out individual, targeted tests, instead of having to complete comprehensive test programmes – i.e. a tailor-made test programme instead of a standard approach. "Among other things, REACH provides for a Daphnia test as an ecotoxicological entrance test", explains Prof. Gerrit Schüürmann. "We have developed a QSAR model that provides decision support for recognising substances for which this test and the associated costs could be economized."
The latest draft of the REACH EU chemicals regulation provides that only chemicals with an annual production of 100 tonnes or more should be intensively
tested using animal experiments. On the other hand, though, all substances that are classed as being of very high concern will be subject to authorisation.
This applies to substances that are carcinogenic, mutagenic or toxic to reproduction; which are persistent, bio-accumulative and toxic; which are very persistent
and very bio-accumulative; and those that have a hormone-like effect. QSAR models can therefore help filter out of the tens of thousands of substances with low
annual production those which are very likely to be subject to authorisation and must therefore be tested in animal experiments. The Danish environment agency,
for instance, has carried out QSAR analyses for around 47 000 organic substances on the European market. In doing so, it established that 20 000 of them fulfil
one or more criteria of a hazardous substance.
In the US, the Environmental Protection Agency has been using QSAR models for over a decade. In Europe these test methods are used to calculate the accumulation
of substances in organisms. They are however rather the exception, since the authorities have tended to be reticent with regard to QSARs. Gerrit Schüürmann
thinks there will be a breakthrough in the next three years: "We have to find a way of placing substances in the context of toxicological knowledge." After all,
the aims of REACH can be achieved only by combining experimental findings with alternative assessment methods like QSARs.
REACH – the new EU chemicals regulation
REACH stands for Registration, Evaluation and Authorisation of Chemicals. The main focus is on ‘old’ chemicals, which were placed on the market before 1981.
The number of chemicals that must be tested in future has been hotly debated. According to the latest draft, about 30 000 of an estimated 100 000 old chemicals
must now be tested. The vast majority are practically untested, despite the fact that EU citizens constantly come into contact with them in everyday life.
"This unequal treatment of so-called old substances, which were taken as given, and new substances, which were strictly regulated, has slowed down innovation",
believes Prof. Bernd Hansjürgens, an economist at the UFZ. By treating all substances equally, it is hoped that the legislation will provide incentives for the
industry to develop more environmentally friendly and healthier substances. In future, the only substances that will be allowed onto the market will be those
for which sufficient data is available. The tests apply to chemicals with a production volume of one tonne per year or more. Another new feature is the shifting
of the burden of proof. In future, companies will have to prove the safety of their products. Until now, substances could be used until the authorities had
evidence that they were harmful. Now companies have to prove that a substance is harmless.
In future REACH should ensure that the whole life of a chemical from producer to consumer can be understood and made safe. Registration is controlled by a new
EU Chemicals Agency in Helsinki. This agency will also decide in individual cases whether particularly hazardous chemicals will receive authorisation. The test
procedures for so-called old chemicals are not expected to be completed before 2017.
More information from:
about computer based models testing chemicals ("QSAR"):
Prof. Gerrit Schüürmann
UFZ, Department Chemical Ecotoxicology
Phone: +49 341 235 2309
alternative test strategies:
Dr. Kristin Schirmer
UFZ-Department Cell Toxicology
Phone: +49 341 235-2699
Dr. Eberhard Küster
UFZ, Department Chemical Ecotoxicology
Phone: +49 341 235 2675
about the innovation effects of REACH:
Prof. Bernd Hansjürgens
UFZ, Department Economics
Phone: +49 341 235 2517
about precautionary principles of REACH:
Prof. Wolfgang Köck
UFZ, Department Environmental and Planning Law
Phone: +49 341 235 3140
or from the
UFZ press office
Doris Böhme, Tilo Arnhold
Phone: +49 341 235 2278
E-mail: presse@ufz.de
