Press release from April 2nd, 2008
New formula for combating the greenhouse gas nitrous oxide
Germany’s Year of Mathematics:
Mathematics provides key to researching climate-friendly wastewater treatment
Halle/S. The cost of treating wastewater contaminated with nitrogen could be lowered in future. Soil scientists at the Helmholtz Centre for Environmental Research (UFZ) have developed a new mathematical model which can help determine the optimum conditions for microbiological water treatment. Using the stable natural nitrogen isotope 15N, this mathematical model, which is the most accurate to date, can for the first time calculate exactly the quantities of dinitrogen (N2) produced by the complex biochemical treatment processes anammox and denitrification and the background levels in the atmosphere, according to the researchers writing in the specialist journal Rapid Communications in Mass Spectrometry. This means that in future the effectiveness of such wastewater treatment plants can be significantly improved and emissions of the greenhouse gas N2O (a by-product of denitrification) can be avoided.

The agriculture is one of the main sources for the greenhouse gas N2O which results from the
excessive use of nitrogenous nutrients.
Source: André Künzelmann/UFZ
Florian Stange and Oliver Spott developed new mathematical equations.
Source: Florian Stange and Oliver Spott/UFZ
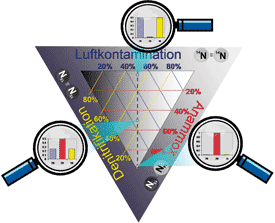
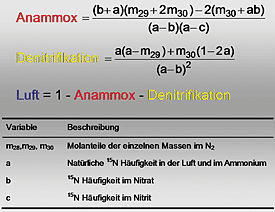
Precise calculation of the quantities of N2 from anammox, from denitrification and from
the atmosphere with the new mathematical equation.
Source: Florian Stange and Oliver Spott/UFZ
As well as the greenhouse gas carbon dioxide (CO2), which has been the subject of much public debate, the less well known nitrous oxide (N2O, also known as ‘laughing gas’) also plays a key role in climate change. As with CO2, a steep rise in atmospheric concentrations of nitrous oxide has been registered since the start of industrialisation. Although the concentration of carbon dioxide in the atmosphere is about 1000 times higher than that of nitrous oxide, nitrous oxide is 300 times more powerful in terms of its greenhouse gas effect than carbon dioxide. In contrast with CO2, the increase in atmospheric N2O concentrations is due only in small part to the combustion of fossil fuels. By far the greatest proportion of the N2O released by humans results from the excessive use of nitrogenous nutrients (such as nitrate/NO3-), which are converted into the greenhouse gas N2O by natural microbial processes (nitrification and denitrification).
One of the key concerns of the European Water Framework Directive (WFD), which came into force in 2000, is the reduction of nitrogen-containing nutrients in waterbodies. One approach is to avoid or optimise the use of nitrogen fertilizers in agriculture. Another approach is to improve wastewater treatment technologies.
Current biological treatment methods are based on the microbial processes of nitrification and denitrification. Although these methods make it possible to treat wastewater containing high levels of nitrogen safely, they also have a considerable disadvantage: the nitrogen that is removed is primarily released into the atmosphere in the form of the greenhouse gas N2O. This is a dilemma in that water protection and climate protection have until now been mutually exclusive.
During experiments with the treatment of nitrogen-contaminated wastewater at the beginning of the 1990s, a previously unknown microbial process was discovered that can break down the main components of the nitrogen contamination in the wastewater (ammonium and nitrate) in the presence of atmospheric oxygen (anaerobically). The only end product is environmentally neutral molecular nitrogen (N2). Exploiting this process, called ‘anammox’, to clean nitrogenous wastewater could in future lead to an entirely climate-neutral treatment of municipal wastewater. In addition, unlike the microbial processes used until now, the anammox process does not require organic nutrients, so in future it will be possible to manage without the addition of nutrients during the treatment process. This will reduce the cost of wastewater treatment still further.
However, particularly with regard to the development of efficient wastewater treatment systems, research into the anammox process is still posing significant problems more than 15 years after it was discovered. The main reason for this is that the end product of the anammox process under investigation (N2) can also be produced simultaneously in the course of the denitrification process, so it has been almost impossible to quantify the conversion rate accurately. In addition, the molecular nitrogen (N2) resulting from the microbiological processes is in principle ‘invisible’ because of the high background concentration of N2 in the atmosphere (≈79 vol. %), since the quantities of N2 released are extremely small compared with the existing atmospheric nitrogen levels. Now for the first time, the two soil scientists Oliver Spott and Florian Stange of the Helmholtz Centre for Environmental Research (UFZ) have succeeded in developing a new mathematical model that can calculate precisely the quantities of N2 from anammox, from denitrification and from the atmosphere. The model is based on analyses using stable isotopes, and means that in future it will be possible to investigate in greater detail the optimum conditions for microbiological treatment of nitrogenous wastewater using the anammox process. This will in turn make it possible to reduce the costs of wastewater treatment in the long term, increase its effectiveness and avoid N2O emissions. The two scientists have published their discovery in the internationally renowned specialist journal Rapid Communications in Mass Spectrometry.
The idea for developing the new mathematical approach using the stable nitrogen isotope 15N came from their collaboration with UFZ colleagues Peter Kuschk and Diego Paredes, who have been looking at the possibility of microbial treatment of nitrogenous wastewater using anammox for a long time. But the new equations are also of great significance for their own work. In 1992 Japanese scientists described for the first time a metabolic process involving soil fungi (Fusarium oxysporum) which is very similar to the process of anaerobic oxidation of ammonium and which was called codenitrification, after the familiar process of denitrification. Despite this, even 15 years after the discovery of codenitrification, scientists still work on the assumption that when nitrogen from the soil is broken down, it is only denitrification that is responsible for the release of molecular nitrogen (N2). Using the 15N isotope technique and the new mathematical approach, it is now possible to calculate precisely the N2 released from the soil during both processes – denitrification and codenitrification. An initial, very recent study by two British scientists has already come to the surprising conclusion that up to 92 per cent of microbially released N2 is attributable to the process of codenitrification. If these initial results can be confirmed and extended to other soils around the world, this would completely change our current understanding of N2 emissions from the soil. The two scientists Oliver Spott and Florian Stange will be using the new equations to tackle this question in collaboration with international scientists.
Publications:
Spott O, Stange CF:
A new mathematical approach for calculating the contribution of anammox, denitrification, and atmosphere to an N2 mixture based on a 15N tracer technique. Rapid Communications in Mass Spectrometry 21 (14), 2007, p.2398-2406.
http://dx.doi.org/10.1002/rcm.3098
Spott O, Russow R, Apelt B, Stange CF:
A 15N-aided artificial atmosphere gas flow technique for online determination of soil N2 release by using the
Zeolithe Köstrolith SX6®.
Rapid Communications in Mass Spectrometry 20 (21), 2006, p. 3267-3274.
http://dx.doi.org/10.1002/rcm.2722
More information:
Prof. Dr. Florian Stange
Helmholtz Centre for Environmental Research - UFZ
phone +49 345-558-5418
Prof. Dr. Florian Stange
Oliver Spott
Helmholtz Centre for Environmental Research - UFZ)
phone +49 345-558-5419
Oliver Spott
oder über
Helmholtz Centre for Environmental Research - UFZ
Press office
Tilo Arnhold / Doris Böhme
phone +49 (0)341 235 2278
presse@ufz.de
Links
Working group Stable Isotopes at the UFZ.:
www.sibc.ufz.de
The Helmholtz Centre for Environmental Research – UFZ was established in 1991 and has about 800 employees in Leipzig, Halle/S. and Magdeburg. They study the complex interactions between humans and the environment in cultivated and damaged landscapes. The scientists develop concepts and processes to help secure the natural foundations of human life for future generations.
The Helmholtz Association contributes to solving major challenges facing society, science and the economy with top scientific achievements in six research areas: Energy, Earth and Environment, Health, Key Technologies, Structure of Matter, Transport and Space. With 25,700 employees in 15 research centres and an annual budget of approximately 2.3 billion euro, the Helmholtz Association is Germany's largest scientific organisation. Its work follows in the tradition of the great natural scientist Hermann von Helmholtz (1821-1894).
